use the shortcut van ’t Hoff equation to determine the flow rate of benzene in the exiting stream.
Benzene can be formed by the dehydrogenation of cyclohexane:
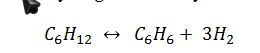
If 1000 mol/s of pure cyclohexane enters a steady state reaction, and the stream leaving the reactor is a vapor phase mixture at P = 0.1 MPa and T = 500 K,
A. 0 mol/s
B. 122 mol/s
C. 1000 mol/s
D. 1366 mol/s
E. The problem cannot be solved.
B. Correct. This is the rate of benzene leaving the reactor if the shortcut van ‘t Hoff assumption is correct.
You might also like to view...
A building earns a LEED rating by:
A) Using LEED-approved materials and contractors. B) Earning points in five different areas. C) Paying for a LEED stamp of certification. D) Following a detailed, prescriptive methods for design and construction.
All of these are types of injector circuits EXCEPT ________
A) Peak width hold B) Saturated switch C) Pulse width modulated D) Peak and hold
The attic is the space formed between the ceiling joists and the rafters.
Answer the following statement true (T) or false (F)
Which of the following is not one of the four basic parts of a legal contract?
A) Offer and Acceptance B) Competent parties C) Execution D) Negotiation