The pH of an acidic solution is a measure of the concentration of the acid particles in the solution, with smaller values of the pH indicating higher acid concentration. In a laboratory experiment, the pH of a certain acid solution is changed by dissolving over-the-counter antacid tablets into the solution. In this experiment, the pH changes according to the equation
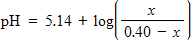
where x is the number of grams of antacid added to the solution. What is the pH of the solution after the addition of 0.05 grams of antacid tablet?
A. 5.99
B. 4.02
C. -0.85
D. 4.29
E. 5.14
Answer: D
Mathematics
You might also like to view...
Solve the system by any method.2y = - 5x - 320x = - 8y - 12
A. {(-15, -6)} B. {(-6, -15)} C. ? D. {(x, y)|5x + 2y = -3}
Mathematics
Draw the next figure in the pattern.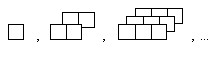
A. ![]()
B. ![]()
C. ![]()
D. ![]()
Mathematics
Find the exact value under the given conditions.sin ? = 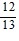 ,
, 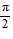 < ? < ?; cos ? =
< ? < ?; cos ? =  , 0 < ? <
, 0 < ? <  Find sin (? - ?).
Find sin (? - ?).
A. - 
B. 
C. 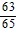
D. 
Mathematics
Find the LCD. Build up the fractions to equivalent fractions having the LCD as the denominator. and
and 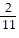
A.  and
and 
B.  and
and 
C. 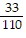 and
and 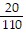
D.  and
and 
Mathematics