The age of a fossil can be estimated by analyzing the decay of radioisotopes within the accompanying rock. If you suspected a fossil was 100 million years old, which type of radioisotopes would you use to analyze the accompanying rock?
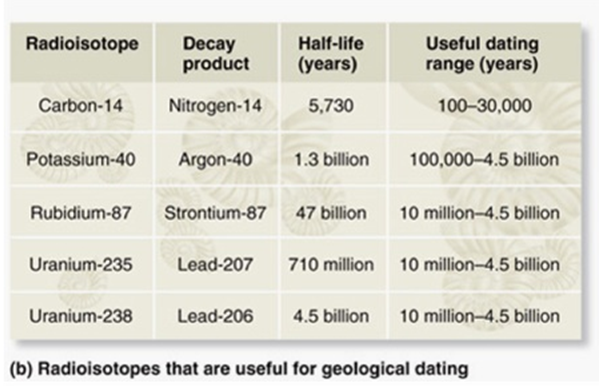
A. Potassium-40/Argon-40 (half-life = 1.3 billion years; useful dating range =100,000-4.5 billion years)
B. Rubidium-87/Strontium-87 (half-life = 47 billion years; useful dating range = 10 million-4.5 billion years)
C. Uranium-235/Lead-207 (half-life = 710 million years; useful dating range = 10 million-4.5 billion years)
D. Carbon-14/Nitrogen-14 (half-life = 5,730 years; useful dating range = 100-50,000 years)
E. Uranium-238/Lead-238 (half-life 4.5 billion years; useful dating range = 10 million-4.5 billion years)
A. Potassium-40/Argon-40 (half-life = 1.3 billion years; useful dating range =100,000-4.5 billion years)
You might also like to view...
Which number identifies a structure that aids in attachment?
a. 1 b. 2 c. 3 d. 7 e. 8
Which situation may result from genetically modifying foods?
A) rice that allows greater synthesis of vitamin A B) calorie-free potato chips C) a tomato that tastes like a melon D) lettuce that doesn't require water to grow
A neutral solution
A) is hydrophobic. B) has a pH of 0. C) has no H+. D) has no OH-. E) has equal amounts of H+ and OH-.
Which environmental factor is most significant for flowering?
A) Light intensity B) Temperature C) Water availability D) Day length E) Nitrogen availability