How much heat must be removed from 456 g of water at 25.0°C to change it into ice at -10.0°C? The specific heat of ice is 2090 J/(kg?K) and the latent heat of fusion of water is 33.5 × 104 J/kg
A)
105 kJ
B)
153 kJ
C)
57.3 kJ
D)
47.7 kJ
E)
210 kJ
E
Physics & Space Science
You might also like to view...
Alien astronomers are using the radial-velocity method to look for planets around our Sun. Which planet are they most likely to detect first?
A. Jupiter B. Neptune C. Mercury D. Earth
Physics & Space Science
Together, which two gases make up 99% of Jupiter's atmosphere?
A) Ammonia and Methane B) Water Vapor and Methane C) Helium and Ammonia D) Hydrogen and Ammonia E) Hydrogen and Helium
Physics & Space Science
The Sun will exhaust its nuclear fuel in about
A) 5000 AD. B) 5 million years. C) 5 billion years. D) 50 billion years.
Physics & Space Science
The horizontal surface on which the objects slide is frictionless. If F = 12 N, what is the tension in string 1?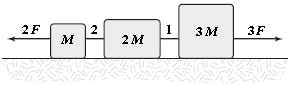
A. 35 N B. 30 N C. 40 N D. 45 N E. 25 N
Physics & Space Science