An electron in the hydrogen atom has a wavelength of 2.67 nm. To what state of the hydrogen atom does this electron belong?
A) n = 2
B) n = 5
C) n = 8
D) n = 11
E) n = 14
C
You might also like to view...
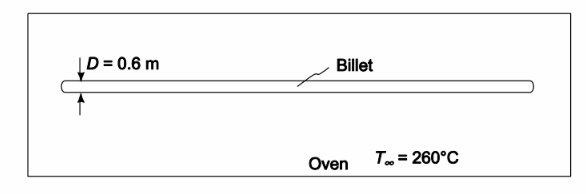
A long 0.6-m-OD 347 stainless steel (k = 14 W/(m K)) cylindrical billet at 16°C room temperature is placed in an oven where the temperature is 260°C. If the average heat transfer coefficient is 170 W/(m2 K), (a) estimate the time required for the center temperature to increase to 232°C by using the appropriate chart and (b) determine the instantaneous surface heat flux when the center temperature is 232°C.
GIVEN
• A long cylindrical billet placed in an oven
• Billet outside diameter = 0.6 m
• Thermal conductivity (k) = 14 W/(m K)
• Initial temperature (Ti) = 16°C
• Oven temperature (T?) = 260°C
• The average heat transfer coefficient h c= 170 W/(m2 K)
• Center temperature increases to 232°C
FIND
(a) The time required using the appropriate chart (b) The instantaneous surface heat fluxes when the center temperature is 232°C
ASSUMPTIONS
• Radial conduction only in billet
• Uniform and constant properties
SKETCH
What is a particle that has no charge and resides in the nucleus of an atom?
A. Isotope B. Neutron C. Electron D. Proton
The part of the eye that distinguishes between different colors of light is the
A. cornea B. lens C. cones D. rods
Why are optical fibers important for communication?
a. They can carry thousands of times the information that wires can. b. Light goes faster than electricity. c. They carry digital information. Wires can't carry digital information. d. all of the above