What happens to the properties of elements across any period of the periodic table?
A) The elements tend to become more metallic in nature since they are increasing in atomic number.
B) The elements get much larger in size because of the addition of more protons and electrons.
C) The properties of the elements gradually change across any period of the periodic table.
D) All of the above are true.
Answer: C
You might also like to view...
A future news release might report that a new planet has been found around a star very similar to our Sun. The system contains other planets as well like our solar system. This newly discovered planet is claimed to have a mass 40 times that of Earth and is located nearly 25 AU from the star it orbits. Which of the following would be a reasonable prediction about this planet, assuming it formed
like our solar system? I. The planet will probably have a mean density of around 5 g/cm3. II. The planet will probably have a radius of around five to ten times greater than Earth's. III. The planet will probably have several satellites. IV. The planet will probably have a composition that is mostly hydrogen and helium. a. I & IV b. I, II, & III c. II, III, & IV d. I, II, & IV e. I, II, III, & IV
The above reactions are of the ___________________
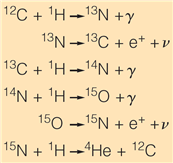
a. proton-proton fusion chain
b. hydrogen-hydrogen fusion chain
c. CNO cycle
d. All of the other choices are correct.
Why does Saturn radiate even more excess energy than Jupiter?
A) Saturn is still radiating heat left over from its formation. B) Saturn's thick cloud layer contributes to a larger Greenhouse Effect. C) Helium rain gives off heat as it differentiates toward Saturn's center. D) Saturn's atmosphere contains much methane, creating a large Greenhouse Effect. E) Saturn can fuse hydrogen into helium in its core, like the Sun.
A room in a house has a floor area of 110 ft2 . Which of the following is most likely the approximate volume of the room?
a. 3 m3 b. 30 m3 c. 300 m3 d. 3 000 m3 e. 10 m3