Using the shortcut van ’t Hoff equation, determine the temperature (in K) at which this reaction will have an equilibrium constant of KT = 1.
Benzene can be formed by the vapor phase dehydrogenation of cyclohexane:
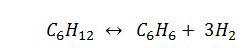
A. 1.02 K
B. 202 K
C. 295 K
D. 568 K
E. Insufficient information to solve.
D. Using data from Appendix C and the shortcut van ‘t Hoff equation produces this answer.
You might also like to view...
A set of cards with words or numbers on either side to be used for classroom drills or private study are often called ____.
a. flipcards b. memory cards c. flashcards d. definitions
?While discussing a pressure test:Technician A says to begin this test by checking line pressure.Technician B says the test can be run with a scan tool and a pressure gauge on transmissions that use an EPC solenoid. Who is correct?
A. ?A only B. ?B only C. ?Both A and B D. ?Neither A nor B
Yearling feeders should never be used to clean up crop residues such as cornfields
Indicate whether the statement is true or false.
One of the ball bearing's disadvantages is that most of the weight of the vehicle is exerted on the bottom of the bearing on a very small surface area of the ball and race.
Answer the following statement true (T) or false (F)