An adiabatic compressor, operating at STEADY-STATE, is used to compress an ideal gas stream from an entering condition of P=0.5 bar and T=300 K to an outlet pressure P=2 bar. If the gas has CP*=4R and the compressor is 80% efficient, find the ACTUAL work added (per mole of gas).
An adiabatic compressor, operating at STEADY-STATE, is used to compress an ideal gas stream from an entering condition of P=0.5 bar and T=300 K to an outlet pressure P=2 bar. If the gas has CP*=4R and the compressor is 80% efficient, find the ACTUAL work added (per mole of gas).
Apply the definition of efficiency for a compressor:
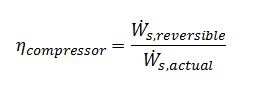
Where W ?_(s,reversible) is obtained by first modeling the compressor as reversible and applying an entropy balance around the compressor to calculate the exiting temperature of the ideal gas (Tout,reversible):
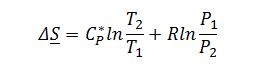
Where ??S is zero since the compressor is modeled as reversible:
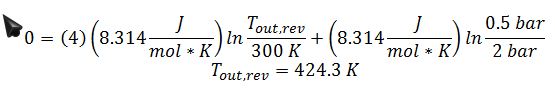
Apply the change in enthalpy of an ideal gas equation to calculate Hout,rev:
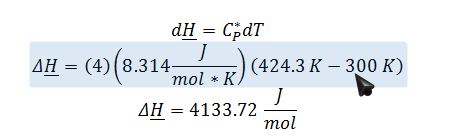
Apply energy balance around the adiabatic reversible compressor to calculate W ?_(s,reversible)

Plug into efficiency equation:
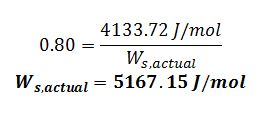
You might also like to view...
When dealing with a plumbing problem, the solution you choose should be the _____.
a. least expensive b. best possible c. most costly d. easiest and fastest
In order to distribute the potential energy created through hydroelectric installations and the burning of coal and oil, these mechanical forms of energy must be converted to ____________________.
Fill in the blank(s) with the appropriate word(s).
What are the results of using undersized piping for headers and uptakes?
What will be an ideal response?
Which character of the VIN indicates the year of the vehicle?
A) First B) Tenth C) Eighth D) None of these