Energy Levels: A doubly-ionized lithium atom Li++, which has 3 protons, undergoes a transition from the n = 5 state to the n = 4 state. What is the wavelength of the photon which is emitted during this transition? (c = 3.00 × 108 m/s, h = 6.626 × 10-34 J ? s, 1 eV = 1.60 × 10-19 J)
A. 350 nm
B. 450 nm
C. 550 nm
D. 650 nm
E. 750 nm
Answer: B
You might also like to view...
The observation that materials expand in size with an increase in temperature can be applied to what proportion of existing substances?
a. 100% b. most c. few d. none
An object moving in a circle with a constant speed has a(n) ______________ directed toward the center of the circle
Fill in the blank(s) with correct word
Length contraction occurs:
a. only along the direction of relative motion. b. only perpendicular to the direction of relative motion. c. in the frame where the moving object is at rest. d. in all directions. e. at a single direction that is unrelated to the direction of relative motion.
Atomic Nucleus: An yttrium isotope has 39 protons and 50 neutrons in its nucleus. Which one of the following symbols accurately represents this isotope?
A. 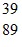 Y
Y
B. 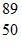 Y
Y
C. 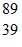 Y
Y
D. 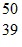 Y
Y
E. 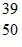 Y
Y