(a) Stirling silver is an alloy of silver with 7.5 percent of copper. Describe the structure that one should expect if a specimen of stirling silver were to be heated from room temperature to 782°C, and allowed to come to equilibrium at this latter temperature.
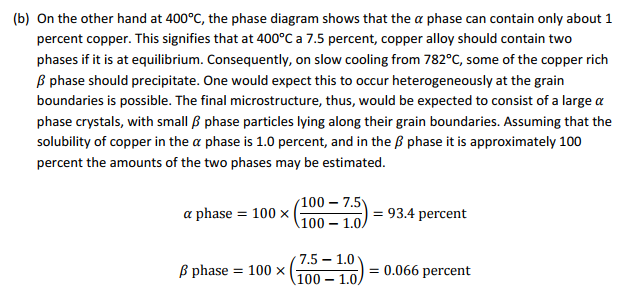
(b) If the specimen of stirling silver, equilibrated at 782°C, is now slowly cooled to 400°C, what would be the nature of the microstructure? Give the amount and composition of each phase.
(c) Finally, assume the specimen is cooled very rapidly (quenched) from 782°C to 400°C. Describe the structure that one might expect.
Solution:
(a) 782°C is only 3° above the eutectic temperature at 779°C. The ? phase extends to 8.8 percent copper at the eutectic temperature. The copper silver phase diagram shows that at 782°C the equilibrium microstructure should be a simply alpha phase solid solution.

You might also like to view...
Accountability is defined as _____.
a. the power to act or make decisions in carrying out assignments b. giving an employee a particular task to perform c. holding an employee responsible for the completng a particular duty d. having the power to promote someone
Tetanus affecting sheep and goats is caused by _____
a. viruses c. insects b. injury d. bacteria
Planting border crops is used in ____________________
Fill in the blank(s) with correct word
Technician A says that an eyewash station should be located in a building corner to keep it clean . Technician B says that an eyewash station should be located in a central area. Which technician is correct?
A) Technician A only B) Technician B only C) Both technicians A and B D) Neither technician A nor B