What was Niels Bohr's explanation for the observation of atomic spectra?
A. Electrons could only move in discrete energy steps within an atom.
B. Only certain photons with the correct energy could excite the quanta in the nucleus.
C. Nucleons could be excited by different electron energies.
D. Any photon could excite an electron.
E. Electrons could not move in an atom.
Answer: A
You might also like to view...
What is being depicted by this figure?
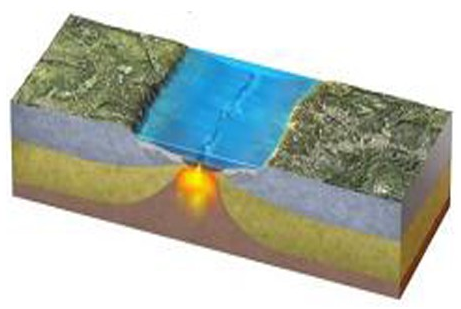
A) Continental collision
B) Continent-continent convergence
C) Early stages of seafloor spreading
D) Early stages of subduction
E) A boundary that is mostly a transform fault
If a neutral element has the following chemical notation, how many electrons does it have? carbon-13
A) 6 B) 12 C) 13 D) 7 E) none of the above
The concept of sustainable development includes
A) the needs of future generations. B) growth in profits from international trade. C) the importance of developing the arts. D) the fastest ways to economic prosperity.
What is the principal cause of septic system failure?
A) plugging of delivery pipes B) poor soil drainage C) failure of advanced treatment systems D) poor tank design