Solve the problem.The hydrogen potential, pH, of a substance is defined by pH = -log [H+], where [H+] is measured in moles per liter. Find the pH of a sample of lake water whose [H+] is 3.05 × 10-9 moles per liter. (Round to the nearest tenth.)
A. 6.4
B. 7.3
C. 10.1
D. 8.5
Answer: D
Mathematics
You might also like to view...
Provide an appropriate response.Find the amount of increase if 500 is increased by 120%.
A. 1,100 B. 100 C. 620 D. 600
Mathematics
Find the difference.-12 - (-1)
A. 11 B. -13 C. 13 D. -11
Mathematics
Provide an appropriate response.Fill in the blanks. 2% represents  parts out of
parts out of  equal parts.
equal parts.
Fill in the blank(s) with the appropriate word(s).
Mathematics
Solve the equation and check.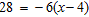
What will be an ideal response?
Mathematics