In a chemical reaction, glyceraldehyde-3-phosphate + NAD+ yields 1,3-bisphosphoglycerate + NADH. In this reaction, what happened to NAD+?
A. It was oxidized to form NADH
B. It was reduced to form NADH
C. It was activated to form NADH
D. it served as an enzyme to catalyze the reaction, and at the end of the reaction formed NADH
Clarify Question
What is the key concept addressed by the question?
What type of thinking is required?
Gather Content
What do you already know about oxidation and reduction chemical reactions? What other information is related to the question?
Choose Answer
Do you have all the information needed to determine the correct answer?
Reflect on Process
Did your problem-solving process lead you to the correct answer? If not, where did the process break down or lead you astray? How can you revise your approach to produce a more desirable result?
B. It was reduced to form NADH
Clarify Question
What is the key concept addressed by the question?
· The question asks you to determine what happens to NAD+ during a chemical reaction.
What type of thinking is required?
· You are being asked to break down, or analyze, the chemical reaction to determine what happens to NAD+.
Gather Content
What do you already know about oxidation and reduction chemical reactions? What other information is related to the question?
· Remember that many chemical processes participate in oxidation and reduction (redox) reactions. Oxidation reactions occur when electrons are liberated from a molecule whereas reduction reactions occur when electrons are added to a molecule. Reduction is frequently associated with addition of hydrogen atoms as well.
· As you may remember, redox reactions are quite common in biochemical pathways such as the reactions described in the question. Given what you know about the participants in redox reactions, what happens to NAD+ when it becomes NADH?
Choose Answer
Do you have all the information needed to determine the correct answer?
· Redox reactions involve one molecule becoming oxidized and another molecule becoming reduced via the removal and addition of electrons, respectively.
· NAD+ is a molecular acceptor of electrons, and is therefore an oxidized molecule. The question indicates that NADH is a product of the reaction. So what is the relationship between NAD+ and NADH?
· When NAD+ accepts electrons, it becomes reduced. The reduced form of NAD+ is therefore NADH.
Reflect on Process
Did your problem-solving process lead you to the correct answer? If not, where did the process break down or lead you astray? How can you revise your approach to produce a more desirable result?
· This question asked you to determine what happens to NAD+ in the reaction.
· If you got the answer correct, good work! If you got an incorrect answer, did you remember how redox reactionsoccur? Were you able to identify which molecules participated in redox reactions? Were you able to discover what happened to NAD+ when it became NADH?
You might also like to view...
In science, if a result is deemed statistically
significant, that means
a. it is a very important result. b. it has a high probability of being incorrect. c. it has a low probability of being skewed by sampling error. d. there is very little variation in the data. e. there is no doubt of the result being true
Refer to Figure 31-5. In which class is this organism placed?
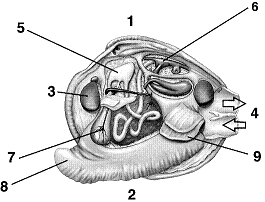
A. Cephalopoda
B. Gastropoda
C. Polyplacophora
D. Bivalvia
E. Chromodoris
What is the significance of the homeodomain?
What will be an ideal response?
In which phase of the cell cycle do cells check to determine whether the DNA is fully and correctly replicated?
(a) at the transition between G1 and S (b) when cells enter G0 (c) during M (d) at the end of G2