If the overall mixture is 20% by mole component 1, will the mixture exist in one phase or multiple phases in equilibrium at 350 K? If the mixture does exist in multiple phases, what is the composition of the equilibrium phases?
Consider the following figure that describes the solid-liquid equilibrium of a binary mixture:
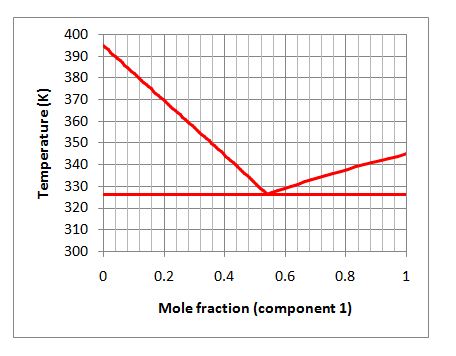
A. 1 liquid phase
B. 1 solid phase
C. 1 liquid phase (x1 = 0.20) and 1 solid phase (z1 = 0.0)
D. 1 liquid phase (x1 = 0.36) and 1 solid phase (z2 = 1.0)
E. 2 solid phases (z1? = 1.0; z2? = 1.0)
A. Incorrect. The point (0.20, 350 K) is below the liquidus, so there must be some solid present.
B. Incorrect. The point (0.20, 350 K) is above the solidus, so there must be some liquid present.
C. Incorrect. You do have one solid and one liquid phase, but are misreading the graph. Redraw your tie line and look at the compositions carefully.
D. Correct. When you draw your tie line at 0.2 and 350K, it intersects the liquidus at 0.36 and the solidus where you have all of component 2 in the solid phase.
E. Incorrect. This occurs only at temperatures less than about 327 K.
You might also like to view...
How can a technician float themselves and safely remove the risk for shock from the hot line?
Fill in the blank(s) with the appropriate word(s).
How is the HP-shape diff erent from the W-shape?
What will be an ideal response?
In binary multiplication, 11 × 11 = ________
A) 1111 B) 11 C) 110 D) 1001
Nonpolarized capacitors _____.
A. must be used with AC only B. must be used with DC only C. can be used with either AC or DC