How many moles of A will be in the reactor at equilibrium?
The gas phase reaction A ? B + C has ?HR0 = 15 kJ/mol and ?GR0 = 10 kJ/mol at 298.15 K. Ten moles of A are placed in a reactor and the system is allowed to come to equilibrium at P = 0.2 MPa and T = 473.15 K. The ideal gas model applies.
A. 0 mol
B. 2.77 mol
C. 7.23 mol
D. 8.05 mol
E. Insufficient information to solve.
C. Applying the shortcut van ‘t Hoff equation and then finding ? to solve for this.
You might also like to view...
The oxygen content of incubators should be maintained at 21%
Indicate whether this statement is true or false.
Determine the number of 4-foot-wide by 8-foot-high sheets of drywall needed for the tenant finish in Figure 16.16. The walls are 8 feet high. The exterior walls need drywall on the interior side only. The interior walls need drywall on both sides. Add 5 percent waste.
What will be an ideal response?
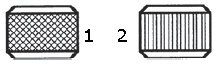 In the accompanying figure, the item identified as number 2 is the ____ pattern.
In the accompanying figure, the item identified as number 2 is the ____ pattern.
A. diamond B. hatch C. hash D. straight line
Using the z-domain-differentiation property find the z transform of 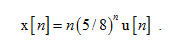
What will be an ideal response?