Using the ideal gas law and the compressibility factor, determine the pressure of methane gas at a temperature of 300 K and specific volumes of (a) 0.005 m3/kg, (b) 0.05 m3/kg, (c) 0.5 m3/kg, and (d) 5.0 m3/kg
Given: CH4, T = 300 K
What will be an ideal response?
For CH4, R = 0.5182 kJ/kg-K
The pressure will be found by the following means:
Ideal Gas Law: P = RT/v
Compressibility factor: P = ZRT/v, where Z is found from the compressibility charts,
using the critical properties of methane: Tc = 191.1 K, Pc = 4640 kPa .
The reduced temperature is TR = T/Tc = 1.57
The pseudo-reduced specific volume is
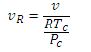
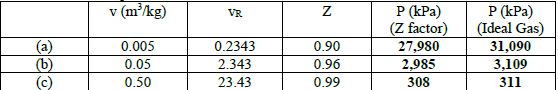
The results for the pressure are as follows:
You might also like to view...
How revolutionary and radical was the New Deal? Did the New Deal reform free market capitalism in substantial ways? Evaluate the significant changes that it wrought, and determine how different the nation became politically, economically, and socially because of it.
What will be an ideal response?
Why are pig heart valves superior to mechanical valves when replacing faulty human heart valves?
What will be an ideal response?
A pedal pulsation condition can be isolated to the front or rear brakes by ________
A) Four hard stops B) Lifting the vehicle slightly C) Using the parking brake to stop D) Rotating the tires
An excavation is a man-made cut, cavity, or trench in the ground made by removing earth
Indicate whether the statement is true or false