This image shows bonding for graphite (on the left) and diamond (on the right). Both minerals are made of carbon, so why do they have very different properties (why is graphite so soft while diamond is so hard)?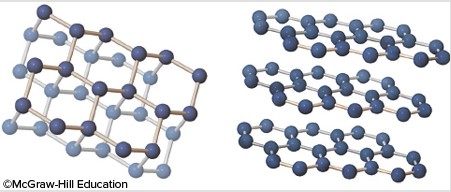
A. Diamond has a different type of covalent bonds between the atoms of carbon than does graphite.
B. Minerals with only one type of bond will always be harder than minerals that have two different types of bonding.
C. Covalent bonds in both minerals are very strong, but intermolecular bonds between sheets in graphite are very weak.
D. Graphite's mixture of covalent and intermolecular bonds is stronger than the solely covalent bonds within diamond.
Answer: C
You might also like to view...
43% of the commercial energy used in the U.S. is unnecessarily wasted.
Answer the following statement true (T) or false (F)
Which ingredient(s) do you think is (are) most likely missing in these
cyclone-free areas? What will be an ideal response?
Layers in sedimentary rocks are called ________
A) foliation B) deposits C) beds D) striations
What is the largest danger to the Russian economy?
a. effects of global warming b. excessive dependence on imports of natural resources c. high percentage of income used to fund the military d. excessive dependence on exports of natural resources e. high unemployment