Exclusion Principle: Write out the electron configuration for the ground state of the phosphorus atom, which has 15 electrons.
Fill in the blank(s) with the appropriate word(s).
1s2 2s2 2p6 3s2 3p3
You might also like to view...
A 10-inch telescope (25.4 cm in diameter) is used to determine if what appears to be one star is actually two stars. Stars are so far away that they are essentially point sources
How close (in angle) can the two stars be and still be resolved by this telescope if it is focusing light of wavelength of 550 nm? (Consider only the limitation due to diffraction.) A) 4.2 × 10-8 degree B) 2.6 × 10-6 degree C) 3.0 × 10-4 degree D) 1.5 × 10-4 degree E) 6.6 × 10-8 degree
What law explains why a collapsing cloud usually forms a protostellar disk around a protostar?
A) conservation of angular momentum B) Kepler's third law C) the universal law of gravitation D) Wein's law
The 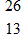 Al nucleus is unstable. To what will it decay?
Al nucleus is unstable. To what will it decay?
A. 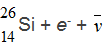
B. 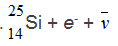
C. 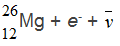
D. 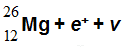
E. Other (specify)
An atom of the element iron has an atomic number of 26 and an atomic mass number of 56. If it is neutral, how many protons, neutrons, and electrons does it have?
A) 26 protons, 30 neutrons, 26 electrons B) 26 protons, 30 neutrons, 30 electrons C) 13 protons, 56 neutrons, 13 electrons D) 13 protons, 43 neutrons, 13 electrons E) 26 protons, 56 neutrons, 26 electrons