Oxygen, O, (number 8), sulfur, S, (number 16), and selenium, Se, (number 34) have such similar chemical properties because
A) their outermost shells contain the same number of electrons.
B) because they are all located close to one another in the periodic table.
C) they all have the same number of occupied shells.
D) These elements can't have similar chemical properties because they are in different periods of the periodic table.
Answer: A
You might also like to view...
Pressure in a Liquid: As shown in the figure, fluid fills a container having several sections. At which of the indicated points is the pressure greatest?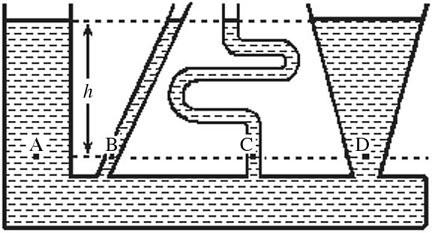
A. A B. B C. C D. D E. The pressure is the same at each of the labeled points.
When a metal surface is illuminated with light of wavelength 437 nm, the stopping potential for photoelectrons is 1.67 V
(c = 3.00 × 108 m/s, h = 6.626 × 10-34 J • s, e = - 1.60 × 10-19 C, 1 eV = 1.60 × 10-19 J, mel = 9.11 × 10-31 kg) (a) What is the work function of the metal, in eV? (b) What is the maximum speed of the ejected electrons?
What is meant by compressibility?
What will be an ideal response?
The strong force is to gluons as the gravitational force is to
A) Z particles. B) quarks. C) photons. D) gravitons. E) neutrinos.