About how big is an atom?
a. too small to measure
b. about a millionth of a meter across
c. about a billionth of a meter across
d. about a trillionth of a meter across
e. none of the above
E
It's about a tenth of a billionth of a meter.
You might also like to view...
Simple Harmonic Motion: A sewing machine needle moves in simple harmonic motion with a frequency of 2.5 Hz and an amplitude of 1.27 cm.(a) How long does it take the tip of the needle to move from the highest point to the lowest point in its travel?(b) How long does it take the needle tip to travel a total distance of 11.43 cm?
What will be an ideal response?
Hubble's constant is related to the age of the universe, but the precise relationship depends on the way in which the expansion rate changes with time. For a given value of Hubble's constant (such as 22 km/s/Mly), the age of the universe is oldest if
A) the expansion rate has been increasing with time (an accelerating universe). B) the expansion rate has remained nearly constant with time (a coasting universe). C) the expansion rate slows ever more gradually with time (a critical universe). D) the expansion rate is slowing enough that it will eventually reverse (a recollapsing universe).
An object’s x-acceleration ax (t) is shown in the boxed graph at the top left. Which of the other graphs in the set most correctly describes its x-velocity?
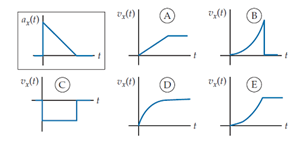
A. A
B. B
C. C
D. D
E. E
Deuterium oxide, ImageO, and water, ImageO, have the same chemical structure and differ only in that ImageO possesses the deuterium isotope of hydrogen, whereas water possesses the protium isotope
Deuterium oxide, also known as "heavy water," is 11 percent heavier than water. Might you expect its boiling temperature also to be about 11 percent greater? Why or why not? A) The mass of the molecules has a far greater influence on the boiling temperature of the substance than does the polarity of the molecules, so ImageO boils at a higher temperature. B) Since they have the same chemical structures, there is a similar molecular attraction between the molecules, so their boiling temperatures are similar. C) Since deuterium is radioactive, it breaks down more easily, so it has a lower boiling temperature than water. D) Since boiling temperature is a measure of the speed of a molecule, the heavier molecules move slower and thus have a higher boiling temperature.