One of the main problems with the Bohr model of the hydrogen atom when compared with the results of the methods of quantum mechanics used to describe atoms, was that the Bohr model predicted
a. the ground state angular momentum was L = 1 .
b. the frequency of the radiation emitted when an electron "jumps" from one allowed orbit to another was hf = Ei ? Ef.
c. the potential energy function for the hydrogen atom was given by V(r) = ?ke2/r.
d. the energy of the ground state of the hydrogen atom was En = ?13.6 eV.
a
You might also like to view...
Photon Energy: A laser emits a pulse of light that lasts 10 ns. The light has a wavelength of 690 nm, and each pulse has an energy of 480 mJ. How many photons are emitted in each pulse? (c = 3.0 × 108 m/s, h = 6.626 × 10-34 J ? s)
A. 1.7 × 1018 B. 2.1 × 1027 C. 2.6 × 1037 D. 3.1 × 1043
Momentum: A 0.10-kg ball, traveling horizontally at 25 m/s, strikes a wall and rebounds at 19 m/s. What is the magnitude of the change in the momentum of the ball during the rebound?
A. 1.2 kg ? m/s B. 1.8 kg ? m/s C. 4.4 kg ? m/s D. 5.4 kg ? m/s E. 72 kg ? m/s
Mineral sample #4 is ________.
Sphalerite - ZnS Apatite - Ca5(PO4)3(F,Cl,OH) Goethite - ?-FeO(OH)
A cart starts with velocity v and rolls up the frictionless path shown. At what point is the kinetic energy greatest?
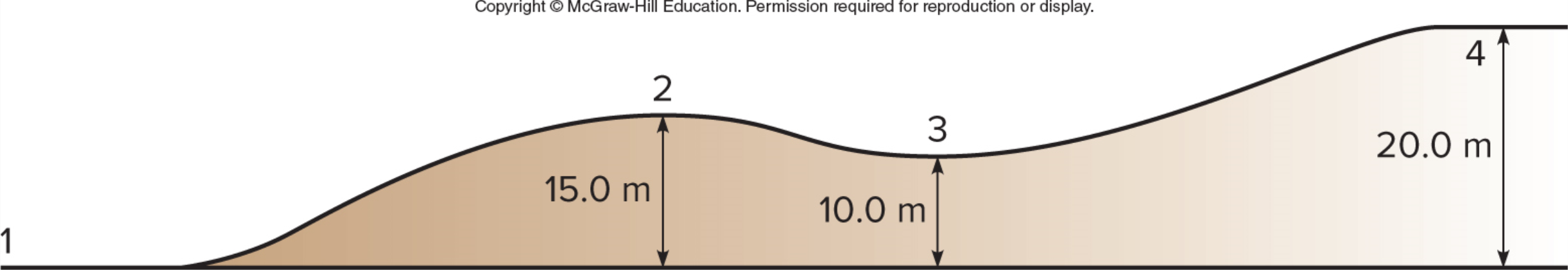
A. 1
B. 2
C. 3
D. 4