Solve the problem.The van der Waals equation provides an approximate model for the behavior of real gases. The equation is P(V, T) = 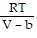 -
- 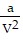 , where P is pressure, V is volume, T is Kelvin temperature, and a,b , and R are constants. Find the derivative of the function with respect to each variable.
, where P is pressure, V is volume, T is Kelvin temperature, and a,b , and R are constants. Find the derivative of the function with respect to each variable.
A. PV =  ; PT =
; PT = 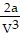 -
- 
B. PV = - 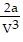 +
+  ; PT =
; PT = 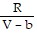
C. PV = 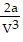 -
- 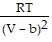 ; PT =
; PT = 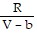
D. PV =  -
- 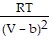 ; PT =
; PT = 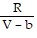
Answer: C
Mathematics
You might also like to view...
Find the Taylor series generated by f at x = a.f(x) = 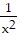 , a = 4
, a = 4
A. 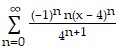
B. 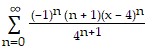
C. 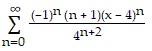
D. 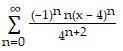
Mathematics
Use the compound-interest formula to find the account balance with the given conditions: P = principal, r = interest rate, t = time, in years.P = $1600, t = 7, r = 9% compounded daily
A. $3003.94 B. $300.39 C. $2997.12 D. $864,584.68
Mathematics
Solve the equation.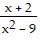 +
+ 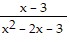 =
= 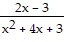
A. 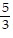
B. 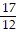
C. 
D. No solutions
Mathematics
Factor out the greatest common factor.64x6y9 + 96x2y4 + 128x4y2
A. 32(2x6y9 + 3x2y4 + 4x4y2) B. No common factor C. 32x2y2(2x4y7 + 3y2 + 4x2) D. 32x2(2x4y9 + 3y4 + 4x2y2)
Mathematics