Argon, which has a constant specific heat as a noble gas, has a specific heat ratio of 1.667 and an ideal gas constant of 0.2081 kJ/kg-K. Determine the values of cp and cv for argon.
Given: k = cp/cv = 1.667; R = 0.2081 kJ/kg-K
What will be an ideal response?
For an ideal gas, cp – cv = R, so cp = R + cv
Substituting: k = (R+cv) / cv = 1.667 = (0.2081 kJ/kg-K + cv)/cv
Solving: cv = 0.312 kJ/kg-K
Then, cp = R + cv = 0.520 kJ/kg-K
You might also like to view...
All materials are elastic to some extent. It is desirable that a part compresses when a load is applied to assist in making an airtight seal (e.g., a jar lid). The results in the following table are from a test conducted at the Smith Test Labs in Seattle on a material known as Zecon 5.
 FIGURE 1.png)
Technician A says that some cabin filters are accessible behind the glove compartment. Technician B says that some cabin filters are accessible from under the hood. Which technician is correct?
A) Technician A only B) Technician B only C) Both technicians D) Neither technician
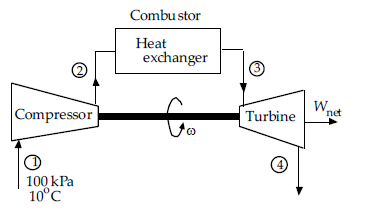
The heat input to the Brayton cycle is nearest:
The pressure and temperature at the inlet to the ideal Brayton cycle shown, are 100 kPa and 10ºC. It has a compression ratio of 8. The maximum temperature in the cycle is 1000ºC. Assume constant specific heats from Table B-2 for all
calculations.
A) 800 kJ/kg
B) 760 kJ/kg
C) 720 kJ/kg
D) 680 kJ/kg
Another way to calculate the minimum number of general lighting circuits required in a dwelling unit is to divide the total habitable square foot living area by ____________ square feet for 15-ampere branch circuits and ______________ square feet for 20-ampere branch circuits.
a. 200/300 b. 600/800 c. 750/1000 d. 1500/2000