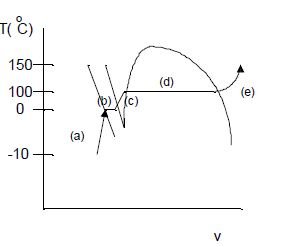
Describe the process of heating water at 1 atm pressure for -10°C to 150°C, at constant pressure. What happens to the water as heat is added? Show the process on a Tv diagram, relating the description to the diagram.
What will be an ideal response?
(a) The water will initially be ice, and the ice will warm from -10°C to 0°C.
(b) At 0°C, the ice will melt until it is all liquid water.
(c) The liquid water will warm, with little volume change, to 100°C.
(d) At 100°C, the water will evaporate into water vapor.
(e) The water vapor will continue to warm to the end point of 150°C. There will be little volume change for parts (a)-(c), but large volume changes for (d) and (e).
You might also like to view...
The male part of the plant is called the
What will be an ideal response?
Over three?quarters of all of the money earned from the meat industry comes from
What will be an ideal response?
The purpose of producing market beef animals is to obtain muscles that can be sectioned into ____________________ cuts of beef for the consumer.
Fill in the blank(s) with the appropriate word(s).
The throttle body may be cleaned (if recommended by the vehicle manufacturer) if what conditions are occurring?
A) Rough idle B) Coast-down stall C) Lower-than-normal idle speed D) Any of the above