This problem concerns a compound for which the following information is available:
• The triple point is located at P=0.8 atm and T=300 K.
• The critical point is located at P=40 atm and T=650 K.
• The acentric factor is 0.25.
• At P=1 atm, boils at 314 K.
• At P=1 atm, the molar volume is 0.12 L/mol for solid and 0.132 L/mol for liquid.
• At the triple point, the enthalpy of fusion/melting is 5000 J/mol.
Give your best estimate of each of the following:
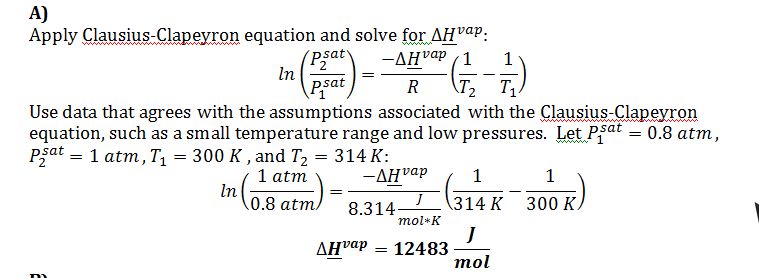
A) The enthalpy of vaporization at the triple point.
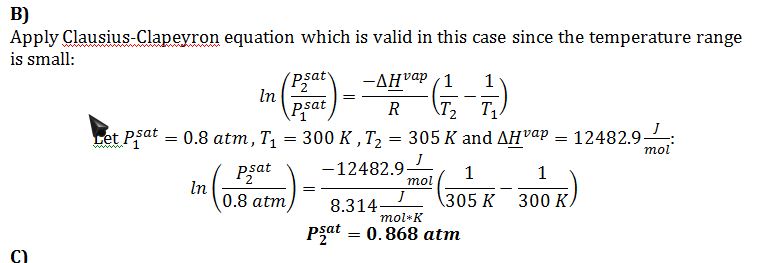
B) The vapor pressure at 305 K.
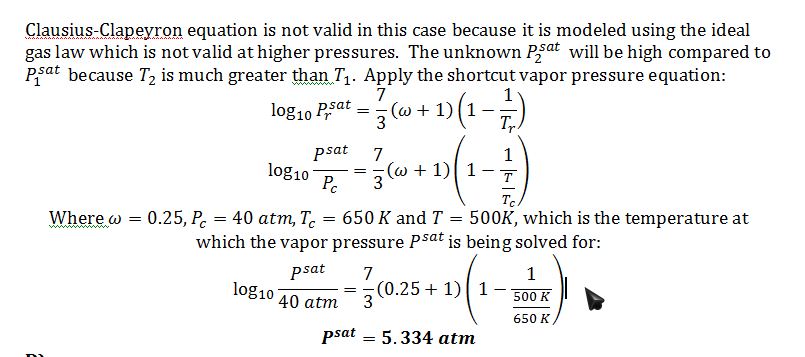
C) The vapor pressure at 500 K.
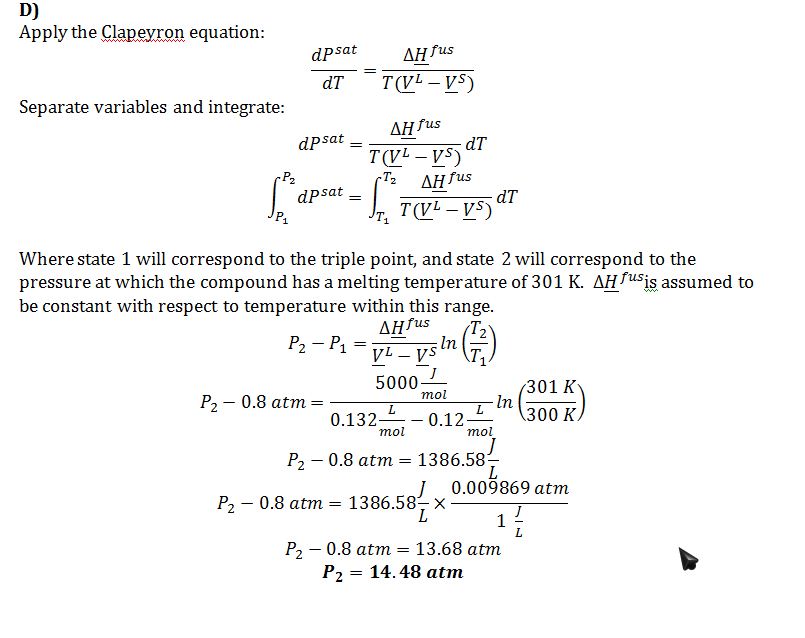
D) The pressure at which the compound has a melting temperature of 301 K.
You might also like to view...
If the foundation plan is not accurate, changes may be required that affect the entire structure.
Answer the following statement true (T) or false (F)
Lynn has a rectangular field 22 chains wide and 38 chains long. Make a scale drawing of her field. Use 1 cm = 2 ch.
What will be an ideal response?
Apple trees do best in cool climates
Indicate whether the statement is true or false
A parallel RLC circuit has an R = 10 k?, an L = 100 mH, and a resonant frequency of 200 krad/s, calculate the value of C, the value of the quality factor, and the bandwidth.
What will be an ideal response?