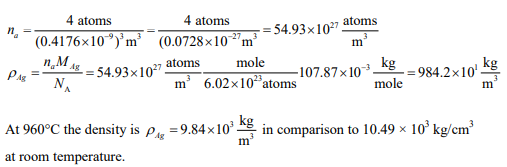
The density of silver at room temperature is 10.49 g/cm3 . You need to know the density of solid silver just below the melting temperature. At 960°C the lattice parameter was measured to be 0.4176 nm. Compare the theoretical density of silver at 960°C to that at room temperature.
What will be an ideal response?
The theoretical density can be calculated from the weight of silver atoms in a single unit cell divided by the volume of a unit cell. Silver is FCC therefore there are 4 atoms per unit cell. The number of atoms per unit volume is:
You might also like to view...
A radioactive isotope with an atomic number of 92 undergoes alpha decay. What is the atomic number of the daughter nucleus?
A) 93 B) 90 C) 91 D) 94
What is the explanation for the pattern of granulation seen on the visible surface of the Sun?
a. The granules form the base of a circulation pattern that extends from the photosphere to the outer corona. b. The granules are regions of nuclear energy generation in the Sun's photosphere. c. Each granule contains a strong magnetic field, which compresses and heats the gas underneath it. d. The granules are the tops of hot gas that have risen from the Sun's convective zone.
Buoyancy: A solid object floats in water with three-fourths of its volume beneath the surface. What is the object's density? The density of water is 1000 kg/m3.
A. 1333 kg/m3 B. 1000 kg/m3 C. 750 kg/m3 D. 250 kg/m3
As the solar nebula contracts due to gravitation, the cloud
A) spins faster. B) expands. C) becomes more spherical in shape. D) changes direction of motion. E) begins to cool.