The ground state electronic configuration for magnesium is 1s22s22p63s2 . Which of the following would most likely result in an element being chemically similar to magnesium?
a. the valence subshell having 2 electrons
b. the valence electron being an s2 electron
c. the valence electron being a p2 electron
d. the valence electron having principal quantum number 3
B
You might also like to view...
A nova destroys the star and leaves behind a white dwarf
Indicate whether the statement is true or false
Some crops are more efficient at utilizing their environments to produce grain growth than others
What will be an ideal response?
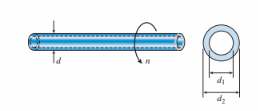
A drive shaft running at 2500 rpm has outer diameter of 60 mm and inner diameter of 40 mm. The allowable shear stress in the shaft is 35 MPa. The maximum power that can be transmitted is approximately:
(A) 220 kW
(B) 240 kW
(C) 288 kW
(D) 312 kW
What atmospheric constituent is responsible for the blue color of Uranus and Neptune?
A) methane B) hydrogen C) water D) ammonia