Consider a vertical 20-cm-tall flat plate at 120°C suspended in a fluid at 100°C. If the fluid is being forced past the plate from above, estimate the fluid velocity for which natural convection becomes negligible (less than 10%) in: (a) mercury (b) air (c) water.
GIVEN
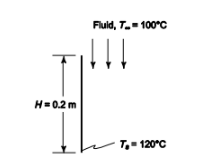
? A vertical flat plate suspended in a fluid
? Plate temperature (Ts) = 120°C
? Fluid temperature (T?) = 100°C
? Fluid is being forced past the plate from above
? Plate height (H) = 20 cm = 0.2 m
FIND
? The fluid velocity (U?) for which natural convection has a less than 10% effect in
(a) mercury (b) air (c) water
ASSUMPTIONS
? Steady state
SKETCH
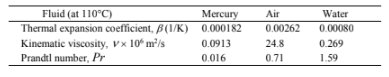
PROPERTIES AND CONSTANTS
From Appendix 2, Tables 26, 28 and 13
From Equation (8.55), for laminar forced convection over a flat plate, the effect of buoyancy will be
less than 10% if
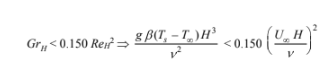
Solving for the fluid velocity
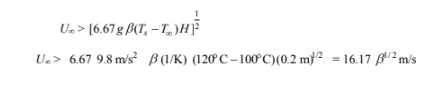

The Reynolds numbers for these fluid velocities are

These Reynolds numbers are all within the laminar regime (mercury is approaching the transition to turbulence). Therefore, the use of Equation (8.55) was valid.
You might also like to view...
The microstructure of the grains of solid ? and ? phases that form in the lead-tin eutectic reaction is _____________ of the ? and ? phases.
Fill in the blank(s) with the appropriate word(s).
On a PV diagram where pressure is in atmospheres and V is in liters the area is measured in liter- atmospheres. What is the number of Joules in 1.00 liter-atmosphere?
A. 101.3 B. 83.1 C. 65.2 D. 22.4 E. 15.7
Latent Heats: How much heat must be added to a 8.0-kg block of ice at -8°C to change it to water at 14°C? The specific heat of ice is 2050 J/kg ? C°, the specific heat of water is 4186 J/kg ? C°, the latent heat of fusion of ice is 334,000 J/kg, and 1 cal = 4.186 J.
A. 780 kcal B. 140 kcal C. 180 kcal D. 810 kcal E. 730 kcal
A boy on a bicycle rides in a circle of radius 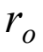 at speed
at speed 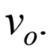 If the boy rides at the same radius
If the boy rides at the same radius 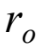 by what factor must he change his speed in order to triple his centripetal acceleration?
by what factor must he change his speed in order to triple his centripetal acceleration?
A. 3.0 B. 0.58 C. 0.33 D. 1.7 E. 0.11 F. 9.0