A beaker of negligible heat capacity contains 456 g of ice at -25.0°C. A lab technician begins to supply heat to the container at the rate of 1000 J/min
How long after starting will the ice begin to melt, assuming all of the ice has the same temperature? The specific heat of ice is 2090 J/kg ? K and the latent heat of fusion of water is 33.5 × 104 J/kg.
23.8 min
You might also like to view...
As a white dwarf cools its radius will not change because
a. pressure due to nuclear reactions in a shell just below the surface keeps it from collapsing. b. pressure does not depend on temperature for a white dwarf because the electrons are degenerate. c. pressure does not depend on temperature because the white dwarf is too hot. d. pressure does not depend on temperature because the star has exhausted all its nuclear fuels. e. material accreting onto it from a companion maintains a constant radius.
Two blocks are accelerated across a horizontal frictionless surface as shown. Frictional forces keep the two blocks from sliding relative to each other, and the two move with the same acceleration. If F = 1.2 N and M = 1.0 kg, what is the horizontal component (frictional force) of the force of the large block on the small block?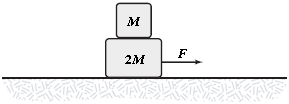
A. 0.40 N to the left B. 0.80 N to the right C. 0.40 N to the right D. 0.80 N to the left E. 1.20 N to the left
What part of a comet always points most directly away from the Sun?
A) the coma B) the jets of gas C) the dust tail D) the plasma tail E) the nucleus
A particle moving along the x axis has a position given by x = (24t – 2.0t3) m, where t is measured in s. What is the magnitude of the acceleration of the particle at the instant when its velocity is zero?
a. 24 m/s2 b. zero c. 12 m/s2 d. 48 m/s2 e. 36 m/s2