A jar holds 2.0 L of ideal nitrogen gas, N2, at STP. The atomic mass of nitrogen is 14.0 g/mol, the ideal gas constant is R = 8.31 J/mol ? K, Avogadro's number is NA = 6.022 × 1023 molecules/mol, and 1.00 atm = 101 kPa
(a) How many moles of nitrogen are in the jar? (b) How many nitrogen molecules are in the jar? (c) What is the mass of the nitrogen in the jar?
(a) 0.089 mol (b) 5.4 × 1022 molecules (c) 2.5 g
You might also like to view...
The lowest energy level an electron can occupy is called the _____
A) ground state B) ionization level C) redshift D) Doppler shift
The diagram illustrates a light source, a gas cloud, and three different lines of sight. Along which line of sight would an observer see an absorption spectrum?
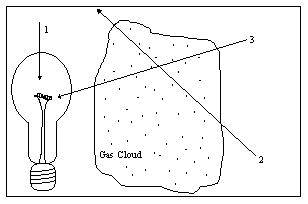
a. 1
b. 2
c. 3
d. 2 and 3
e. none of them
How did Harlow Shapley conclude that the Sun was not in the center of the Milky Way Galaxy?
A) by looking at the shape of the "milky band" across the sky B) by mapping the distribution of stars in the galaxy C) by mapping the distribution of globular clusters in the galaxy D) by mapping the distribution of gas clouds in the spiral arms E) by looking at other nearby spiral galaxies
In a double-slit experiment, the distance between the slits is 0.2 mm and the distance to the screen is 100 cm. What is the phase difference (in degrees) between the waves from the two slits arriving at a point 5 mm from the central maximum when the wavelength is 400 nm? (Convert your result so the angle is between 0 and 360°.)
A. 90° B. 180° C. 270° D. 360° E. 160°