How is the number of unpaired valence electrons in an atom related to the number of bonds that the atom can form?
A. The number of unpaired valence electrons in an atom is twice the number of bonds that the atom can form.
B. The number of unpaired valence electrons in an atom is one-half the number of bonds that the atom can form.
C. There is no defined relationship between the number of unpaired valence electrons and number of bonds that the atom can form.
D. The number of unpaired valence electrons in an atom is the same as the number of bonds that the atom can form.
Answer: D
You might also like to view...
Give both the Manhattan and Euclidean distances between the two points defined on a spatial grid as (1,9) and (5,2)
The following values represent the salaries paid by a university department to all of its employees, omitting units: 27, 60, 143, 25, 24, 27, 42, 98, 29, 30, 97, 59, 26, 43, 27, 44, 25, 27, 26, 61, 145, 26, 40, 27, 46, 28, 24 What will be an ideal response?
Atlanta, Georgia—label the weather conditions you think are occurring. Describe the dominant air mass and relative humidity.
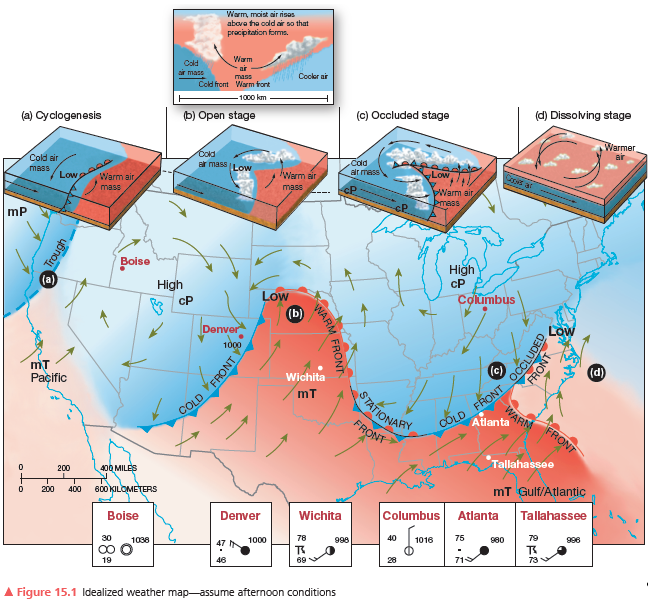
What will be an ideal response?
How do barrier islands form? What are the hazards associated with them? Why is it generally unwise for humans to inhabit them?
What will be an ideal response?
Observe the desert and steppe climates on Figure 20.8 below. How are these climates distributed relative to latitude? What is the relationship of dry climates to global circulation discussed in chapter 18?
What will be an ideal response?