A closed, nonconducting, horizontal cylinder is fitted with a nonconducting, frictionless, floating piston that divides the cylinder into Sections A and B. The two sections contain equal masses of air, initially at the same conditions, 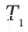 = 300 K and
= 300 K and 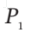 = 1(atm). An electrical heating element in Section A is activated, and the air temperatures slowly increase:
= 1(atm). An electrical heating element in Section A is activated, and the air temperatures slowly increase: 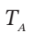 in Section A because of heat transfer, and
in Section A because of heat transfer, and 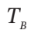 in Section A because of heat transfer, and
in Section A because of heat transfer, and
src="https://sciemce.com/media/4/7bc03e91afc40c5.png" class="w-image" /> in Section A because of heat transfer, and 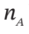 be the number of moles of air in Section A. For the process as described, evaluate one of the following sets of quantities:
be the number of moles of air in Section A. For the process as described, evaluate one of the following sets of quantities:
What will be an ideal response?
(a) 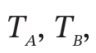 and
and 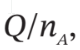 if P(final) = 1.25(atm)
if P(final) = 1.25(atm)
(b) 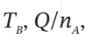 and P (final), if
and P (final), if 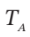 = 425 K
= 425 K
(c) 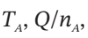 and P (final), if
and P (final), if 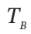 = 325 K
= 325 K
(d) 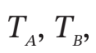 and P (final), if
and P (final), if 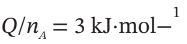
We want to develop relationships between 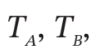 p, and
p, and 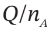 . Then, given any one of the quantities, we can find the other three. One relationship is that the total volume remains constant:
. Then, given any one of the quantities, we can find the other three. One relationship is that the total volume remains constant:
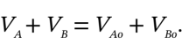 We can rewrite this in terms of the number of moles in each chamber (
We can rewrite this in terms of the number of moles in each chamber (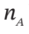 ), the pressure (which is the same in both chambers) and the temperature in each chamber:
), the pressure (which is the same in both chambers) and the temperature in each chamber:
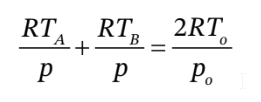
Another relationship arises from the fact that the compression in chamber B is adiabatic, so
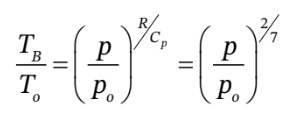
The third relationship derives from the energy balances on the two closed systems. If we let 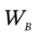 be the work done by the gas in chamber A on the gas in chamber B, then
be the work done by the gas in chamber A on the gas in chamber B, then
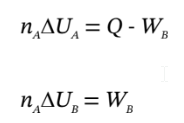
and 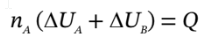
Writing the internal energy changes in terms of the heat capacity and temperature changes:
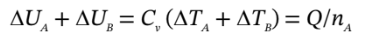
or
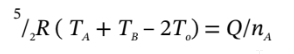
Now, we can apply these three relationships to solve the problems posed in parts (a) through (d). You only had to do one part of your choice.
a. If we know the final pressure, then we can first use:
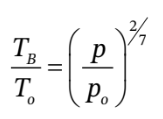
from which 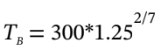 = 319.75 K
= 319.75 K
Next, we can use
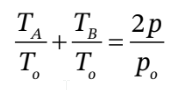
to get
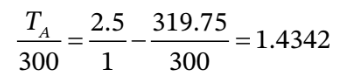
from which 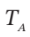 = 430.3 K
= 430.3 K
Finally, then, 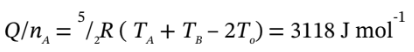
b. This is a bit trickier. One way to approach it is to substitute 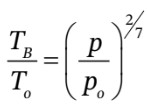 into
into 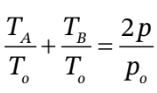 to get
to get 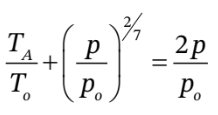
We can then guess values of 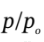 until we find one that gives the specified value of
until we find one that gives the specified value of 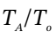 = 425/300 = 1.417. If
= 425/300 = 1.417. If
we want to iterate on the equation, we might write it as:
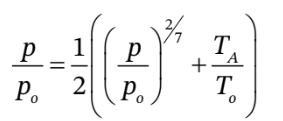
From part (a), we know that 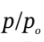 will be close to 1.25. So, we might iterate on the above starting from a guess of
will be close to 1.25. So, we might iterate on the above starting from a guess of 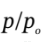 = 1.25.
= 1.25.
This quickly converges to 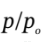 = 1.24, or p = 1.24 *1 atm = 1.24 atm.
= 1.24, or p = 1.24 *1 atm = 1.24 atm.
Now, we can use this directly to get 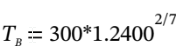 = 319.0 K.
= 319.0 K.
Finally, the two temperatures are used to get 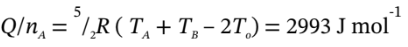
c. This one is a bit more straightforward using our equations. First, we can use 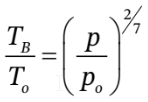 to get
to get 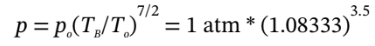 = 1.3233 atm.
= 1.3233 atm.
Then, we can use
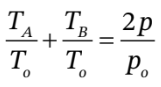 to get
to get 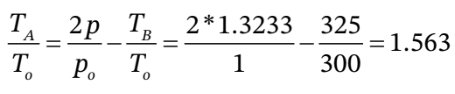
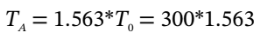
from which 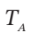 = 469 K.
= 469 K.
Finally, the two temperatures are used to get 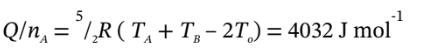
d. This time, we have to start with 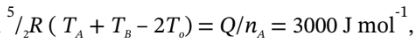 from which
from which 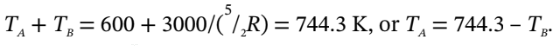 Substituting this into
Substituting this into 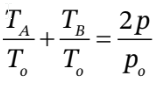 gives
gives 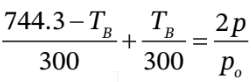 , or just
, or just 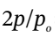 = 744.3/300 = 2.481, from which p = 1.2405 atm.
= 744.3/300 = 2.481, from which p = 1.2405 atm. 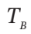 happened to conveniently cancel out.
happened to conveniently cancel out.
Then we can use
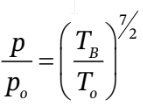 to get
to get 
Finally, 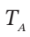 = 744.3 –
= 744.3 – 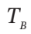 = 425.2 K.
= 425.2 K.
Trades & Technology
src="https://sciemce.com/media/4/7bc03e91afc40c5.png" class="w-image" /> in Section A because of heat transfer, and 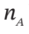 be the number of moles of air in Section A. For the process as described, evaluate one of the following sets of quantities:
be the number of moles of air in Section A. For the process as described, evaluate one of the following sets of quantities:
What will be an ideal response?
(a) 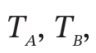 and
and 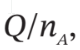 if P(final) = 1.25(atm)
if P(final) = 1.25(atm)
(b) 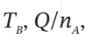 and P (final), if
and P (final), if 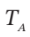 = 425 K
= 425 K
(c) 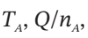 and P (final), if
and P (final), if 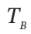 = 325 K
= 325 K
(d) 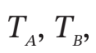 and P (final), if
and P (final), if 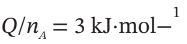
We want to develop relationships between 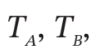 p, and
p, and 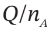 . Then, given any one of the quantities, we can find the other three. One relationship is that the total volume remains constant:
. Then, given any one of the quantities, we can find the other three. One relationship is that the total volume remains constant:
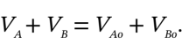 We can rewrite this in terms of the number of moles in each chamber (
We can rewrite this in terms of the number of moles in each chamber ( ), the pressure (which is the same in both chambers) and the temperature in each chamber:
), the pressure (which is the same in both chambers) and the temperature in each chamber:
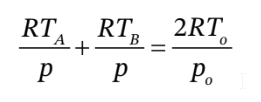
Another relationship arises from the fact that the compression in chamber B is adiabatic, so
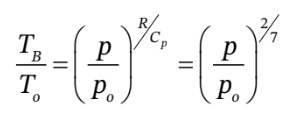
The third relationship derives from the energy balances on the two closed systems. If we let 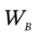 be the work done by the gas in chamber A on the gas in chamber B, then
be the work done by the gas in chamber A on the gas in chamber B, then
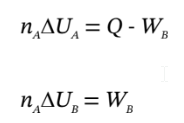
and 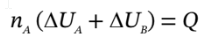
Writing the internal energy changes in terms of the heat capacity and temperature changes:
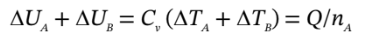
or
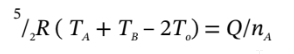
Now, we can apply these three relationships to solve the problems posed in parts (a) through (d). You only had to do one part of your choice.
a. If we know the final pressure, then we can first use:
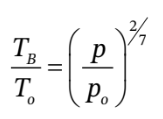
from which 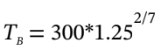 = 319.75 K
= 319.75 K
Next, we can use
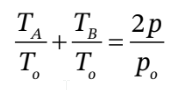
to get
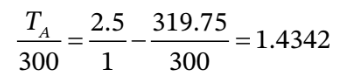
from which 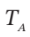 = 430.3 K
= 430.3 K
Finally, then, 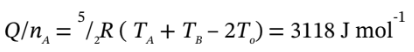
b. This is a bit trickier. One way to approach it is to substitute 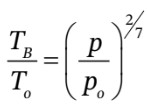 into
into 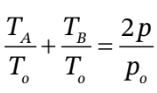 to get
to get 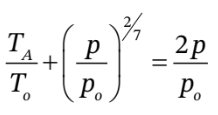
We can then guess values of 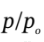 until we find one that gives the specified value of
until we find one that gives the specified value of 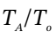 = 425/300 = 1.417. If
= 425/300 = 1.417. If
we want to iterate on the equation, we might write it as:
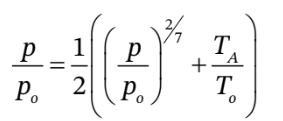
From part (a), we know that 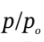 will be close to 1.25. So, we might iterate on the above starting from a guess of
will be close to 1.25. So, we might iterate on the above starting from a guess of 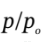 = 1.25.
= 1.25.
This quickly converges to 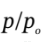 = 1.24, or p = 1.24 *1 atm = 1.24 atm.
= 1.24, or p = 1.24 *1 atm = 1.24 atm.
Now, we can use this directly to get 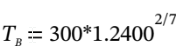 = 319.0 K.
= 319.0 K.
Finally, the two temperatures are used to get 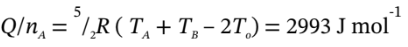
c. This one is a bit more straightforward using our equations. First, we can use 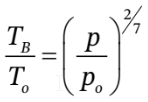 to get
to get 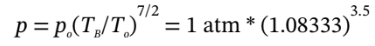 = 1.3233 atm.
= 1.3233 atm.
Then, we can use
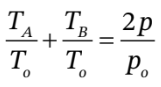 to get
to get 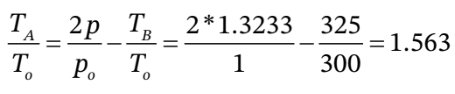
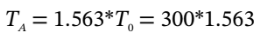
from which 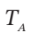 = 469 K.
= 469 K.
Finally, the two temperatures are used to get 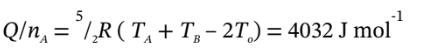
d. This time, we have to start with 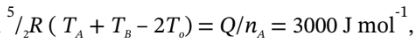 from which
from which 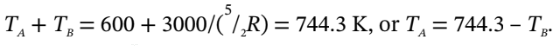 Substituting this into
Substituting this into 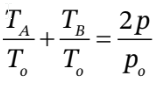 gives
gives 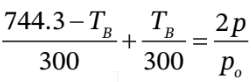 , or just
, or just 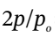 = 744.3/300 = 2.481, from which p = 1.2405 atm.
= 744.3/300 = 2.481, from which p = 1.2405 atm. 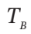 happened to conveniently cancel out.
happened to conveniently cancel out.
Then we can use
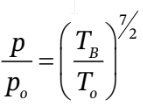 to get
to get 
Finally, 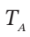 = 744.3 –
= 744.3 – 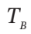 = 425.2 K.
= 425.2 K.
You might also like to view...
Which of the following failures would most likely illuminate the MIL lamp?
A. Low engine oil pressure B. Low engine coolant level C. Disconnected DEF dosing injector connector D. All of the above would likely illuminate the MIL lamp.
Legumes, plants that can take nitrogen from the air, include bluegrass, timothy, redtop, and fescues
Indicate whether the statement is true or false.
To read a dial caliper, ________ the reading on the dial to the reading on the blade
A) Subtract B) Add C) Multiply D) None of these
Using Table 46-2, what width should 2 inch fiberglass duct insulation be to wrap an 8 inch diameter round duct?
What will be an ideal response?