When a neutron and proton combine to form a deuteron, a 2.2 million electron volt gamma ray is emitted. What does this indicate about the binding energy of the deuterium nucleus?
a. Its binding energy is negative, i.e., it is unstable.
b. It has a small binding energy.
c. Its binding energy is about 2.2 million electron volts.
d. The deuterium nucleus is slightly more massive than the sum of the proton and neutron masses, because of energy mass equivalence.
e. The deuterium nucleus contains 2.2 million electron volts more energy than the separated neutron and proton.
c
You might also like to view...
An 8000-kg aluminum flagpole 100-m long is heated by the Sun from a temperature of 10°C to 20°C. Find the heat transferred (in J) to the aluminum if the specific heat of aluminum is 0.215 cal/g/°C
a. 7.2 × 10^5 b. 7.2 × 10^7 c. 7.2 × 10^3 d. 7.2 × 10^1 e. 7.2 × 10^2
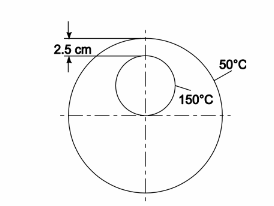
Determine the rate of heat transfer per unit length from a 5-cm-OD pipe at 150°C placed eccentrically within a larger cylinder of 85% Magnesia wool as shown in the sketch. The outside diameter of the larger cylinder is 15 cm and the surface temperature is 50°C.
GIVEN
A pipe placed eccentrically within a larger cylinder of 85% Magnesia wool as shown in the sketch
Outside diameter of the pipe (Dp) = 5 cm = 0.05 m
Temperature of the pipe (Ts) = 150°C
Outside diameter of the larger cylinder (Do) = 15 cm = 0.15 m
Temperature of outer pipe (To) = 50°C
FIND
The rate of heat transfer per meter length (q)
ASSUMPTIONS
Two dimensional heat flow (no end effects)
The system is in steady state
Uniform thermal conductivity
SKETCH
What was Avogadro's hypothesis?
a. That under all conditions each liter of any gas contains the same number of molecules. b. That under identical conditions each liter of all gases contains the same number of molecules. c. That under ideal conditions each liter of all gases contains the same number of atoms. d. That under identical conditions each liter of a particular gas contains the same number of molecules. e. That under identical conditions each liter of all gases contains the same number of atoms.
Solar energy
a. is a recent invention b. has been used for a long time c. has not yet been successfully developed d. is indirectly the source of most of the energy we use