A balloon initially contains air at 25oC and 450 kPa, with a volume of 0.10 m3. The air inside the balloon is heated and the balloon expands until the volume is 0.25 m3. The pressure and volume follow the relationship PV1.3 = constant during the process. Determine the final temperature of the air, and the amount of heat added.
Given: Air; T1 = 25oC = 298 K; P1 = 450 kPa; V1 = 0.10 m3; V2 = 0.25 m3; PV1.3 = constant
Assume: ?KE = ?PE = 0. The air is an ideal gas with constant specific heats (cv = 0.718 kJ/kg-K; R = 0.287 kJ/kg-K)
What will be an ideal response?
Solution: This is a closed system, and the First Law will reduce to
Q – W = m (u2 – u1)
The work is moving boundary work, with the process being polytropic with n = 1.3:
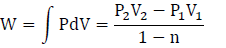
P2 = P1V11.3 / V21.3 = 136.7 kPa
W = 36.05 kJ
m = P1V1/RT1 = 0.526 kg
T2 = P2V2/mR = 226.3 K
Q = m cv (T2 – T1) + W = 8.98 kJ
You might also like to view...
When preparing for the project, the contractor begins the documentation process by keeping logs and schedules for product submittals.
Answer the following statement true (T) or false (F)
A _______ oil pump is used in the majority of four-stroke motorcycle engines.
A. gear B. plunger C. centrifugal D. trochoid
When there is high resistance in a battery cable or its connections, they will become hot to the touch.
Answer the following statement true (T) or false (F)
Water-cooled chillers must have water treatment to ____.
A. increase the pH content of the water in the sump B. decrease the pH content of the water in the sump C. prevent minerals and algae from forming in the water circuit D. help preheat the water in the condenser water circuit