Solve the problem.The Van der Waals equation provides an approximate model for the behavior of real gases. The equation is P(V, T) = 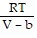 -
- 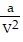 , where P is pressure, V is volume, T is Kelvin temperature, and a,b , and R are constants. Find the partial derivative of the function with respect to each variable.
, where P is pressure, V is volume, T is Kelvin temperature, and a,b , and R are constants. Find the partial derivative of the function with respect to each variable.
A. PV = 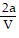 -
- 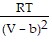 ; PT =
; PT = 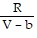
B. PV = 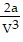 -
- 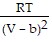 ; PT =
; PT = 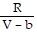
C. PV = 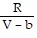 ; PT =
; PT = 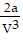 -
- 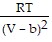
D. PV = - 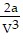 +
+ 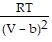 ; PT =
; PT = 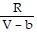
Answer: B
Mathematics
You might also like to view...
Graph the pair of parametric equations with the aid of a graphing calculator.x = 4 sin t - sin 4t, y = 4 cos t - cos 4t, 0 ? t ? 2?
A. 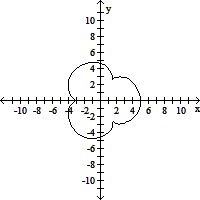
B. 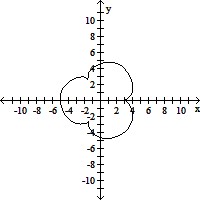
C. 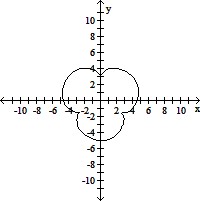
D. 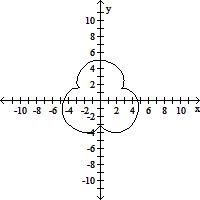
Mathematics
Solve the equation.5z + 18 = 4z + 2
A. 20 B. -20 C. -16 D. 16
Mathematics
Graph the given number, its negative, and its complex conjugate one the coordinate grid.-3 - 5j
A. 
B. 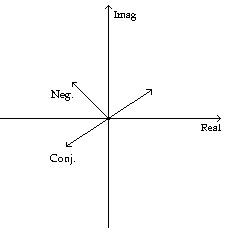
C. 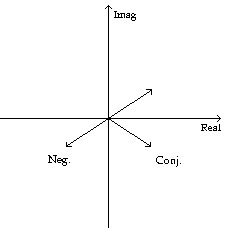
D. 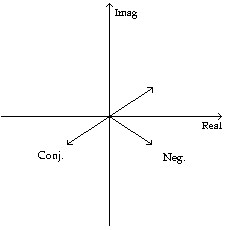
Mathematics
Solve the problem.Shown is the image of a figure under the two successive translations that take P to P' and then P' to P''. Find the original figure. Note again, the given figure is the image after translation. Hint: First find the final image of a point if it were translated by both translations. Then what happens in the reverse process?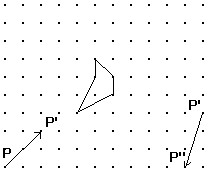
A. 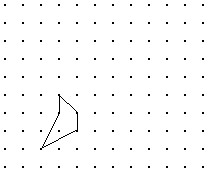
B. 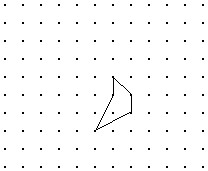
C. 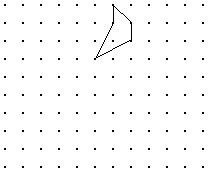
D. 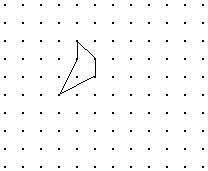
Mathematics