Find the change in enthalpy when 500 grams of the compound are heated at atmospheric pressure from liquid at 50 °C to vapor at 150 °C.
A compound has the following properties at atmospheric pressure:
• Normal boiling point of 95 °C
• Specific enthalpy of vaporization of 900 J/g
• In the liquid phase, CP = 2.4 J/gK
• In the vapor phase, CP = 3.0 J/gK
1 = liquid at 50 °C and 1 atm
2 = saturated liquid at 95 °C and 1 atm
3 = saturated vapor at 95 °C and 1 atm
4 = vapor at 150 °C and 1 atm
Enthalpy is a state property, so:
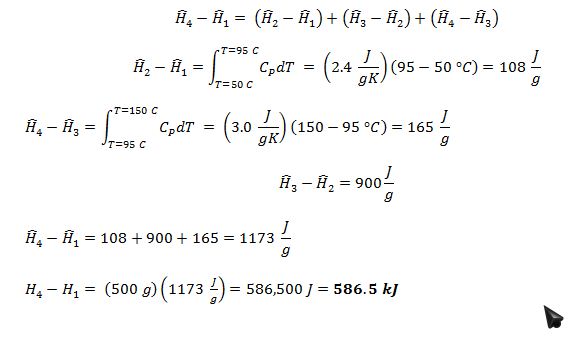
You might also like to view...
Twenty inches of vacuum would be equivalent to how much pressure absolute?
A. 10 psia B. 5 psia C. 0 psia D. 14.7 psia
List the three most common conditions that may cause detonation.
What will be an ideal response?
The European style of floral design is known as ____________________ arrangements
Fill in the blank(s) with correct word
Producers are increasingly using synthetic lines that are derived by ____________________ breeds.
Fill in the blank(s) with the appropriate word(s).