Consider the following data at 298.15 K: Psat (1) = 50 kPa; Psat (2) = 150 kPa. If the liquid behaves as an ideal solution, what can you say about the properties of this mixture?
A. When the liquid is very rich in component (2), the bubble-point pressure approaches 50 kPa.
B. The azeotrope for this system will occur when the system is equimolar.
C. The bubble-point pressure of an equimolar mixture will be 100 kPa at 298.15 K.
D. The system likely has an excess molar enthalpy in the 50 to 150 J/mol range.
E. All of the above are true.
A. Incorrect. When the liquid is rich (highly concentrated) in component 2, the bubble-point pressure should be close to 150 kPa.
B. Incorrect. Ideal solutions do not form azeotropes.
C. Correct. The bubble-point pressure from an ideal solution is a straight line between the two pure component vapor pressures. At x1 = x2 = 0.5, this is (50 kPa + 150 kPa)/2 = 100 kPa
D. Incorrect. If the solution is an ideal solution, then the excess enthalpy is zero for all compositions.
E. Incorrect. One statement is true.
You might also like to view...
Task lighting is used to _____.
a. emphasize art or décor b. illuminate a vertical surface c. focus illumination on a specific surface d. provide minimum exit lighting
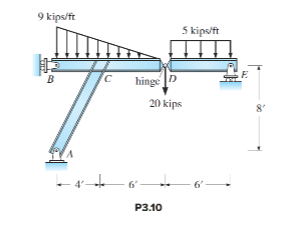
Determine the reaction for the structure in P3.10. All dimensions are measured from the centerlines of members.
What is the central question of Polybius’s forty-book history?
a. How did a single city subdue an entire world? b. How did the people of Italy come to believe it was their destiny to conquer Carthage? c. How could the Greeks persuade the Romans to adopt Greek civilization? d. Why did the Romans abandon their republican institutions in favor of an empire?
More than one signal can be carried by a radio wave using a process called ________
A) Frontband operation B) Frequency Modulation C) Amplitude Modulation D) Sideband operation