A 2.0-kg block of aluminum at 50°C is dropped into 5.0 kg of water at 20°C and the temperature is allowed to stabilize
What is the total change in entropy during this process, assuming no heat is exchanged with the environment? The specific heat of aluminum is 910 J/(kg?K) and the specific heat of water is 4190 J/(kg?K). A) 8.2 J/K
B) 10 J/K
C) 3.3 × 10-2 J/K
D) 3.8 × 10-3 J/K
E) 2.4 × 10-3 J/K
A
You might also like to view...
The specific of ice is 2.10 kJ/kg  the heat of fusion for ice at 0
the heat of fusion for ice at 0 is 333.7 kJ/kg, the specific heat of water 4.186 kJ/kg
is 333.7 kJ/kg, the specific heat of water 4.186 kJ/kg and the heat of vaporization of water at 100
and the heat of vaporization of water at 100 is 2,256 kJ/kg. What is the final equilibrium temperature when 10.0 grams of ice at -15
is 2,256 kJ/kg. What is the final equilibrium temperature when 10.0 grams of ice at -15 is mixed with 40.0 grams of water at
is mixed with 40.0 grams of water at  ?
?
A. 59.5
B. 57.2
C. 53.6
D. 48.9
E. 42.6
Two spaceships are approaching one another, each at a speed of 0.28c relative to a stationary observer on Earth. What speed does an observer on one spaceship record for the other approaching spaceship?
What will be an ideal response?
Kirchhoff's Rules: A multiloop circuit is shown in the figure, but some quantities are not labeled. Find the current I1 if the batteries are ideal. (It is not necessary to solve the entire circuit.)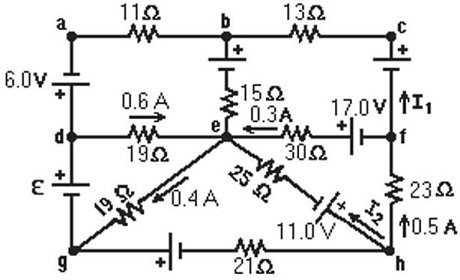
A. 0 A B. +0.2 A C. +0.4 A D. -0.2 A E. -0.4 A
Electromagnetic waves
A. need a medium such as water to propagate B. travel faster in a medium such as water than they do in a vacuum C. are longitudinal waves D. travel at the same speed in a vacuum regardless of wavelength