Ideal Gas Law: A sealed container holds 0.020 moles of ideal nitrogen (N2) gas, at a pressure of 1.5 atm and a temperature of 290 K. The atomic mass of nitrogen is 14.0 g/mol. How many molecules of nitrogen are in the container? (R = 8.31 J/mol ? K, 1 atm = 101 kPa)
A. 1.5 × 1021 mol
B. 3.0 × 1021 mol
C. 6.0 × 1021 mol
D. 1.2 × 1022 mol
E. 2.4 × 1022 mol
Answer: D
You might also like to view...
_________are used to determine temperature based upon the electrical resistivity of a material.
Fill in the blank(s) with the appropriate word(s).
Two mirrors are joined together along a common side, the planes of the mirrors separated by the angle ? . If the joined mirrors can be used as a retroreflector when a beam of light strikes both surfaces (one after the other), what is the value of ??
a. 30° b. 45° c. 90° d. 135°
Graphical Analysis: Which of the following graphs represent an object having zero acceleration?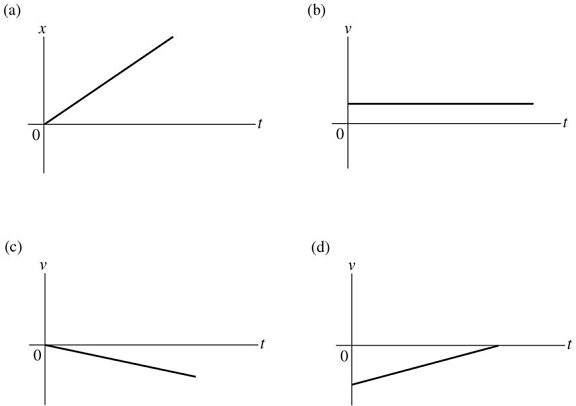
A. only graph a B. only graph b C. graphs a and b D. graphs b and c E. graphs c and d
There are no environmental drawbacks to wind energy
What will be an ideal response?