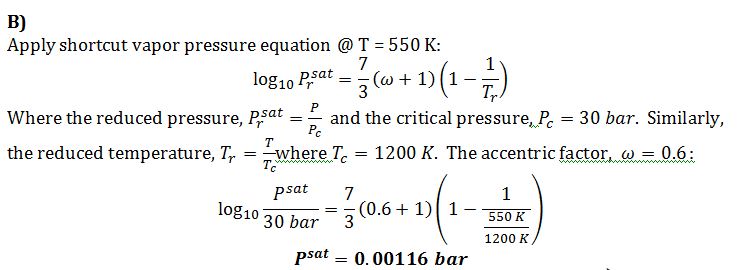
This problem concerns a compound for which the following data is available:
Critical Temperature = 1200 K
Critical Pressure = 30 bar
Enthalpy of Vaporization at atmospheric pressure = 5000 J/mol.
Boiling point at one bar of pressure = 500 K
Acentric factor = 0.6
Equation of state for gas/vaporphase: Z = 1 + bP2/T where b = 1 K/bar2
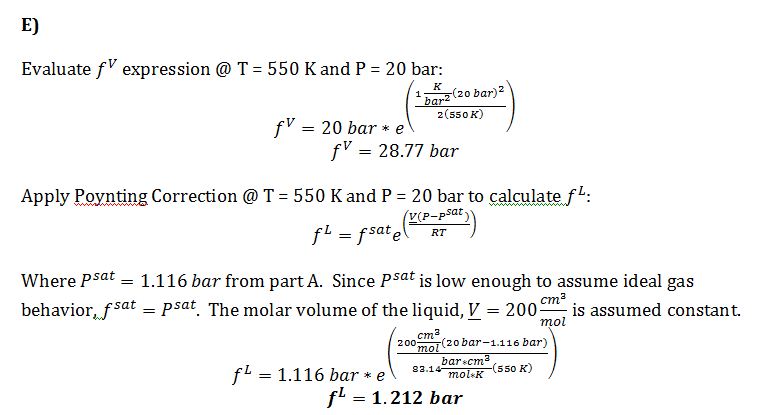
Approximate molar volume of liquid = 200 cm3/mol
A) Use the Claussius-Clapeyron Equation to estimate the vapor pressure at T=550 K.
B) Use the shortcut equation to estimate the vapor pressure at T=550 K.
C) Indicate which of the two estimates generated in questions A and B is more reliable, and EXPLAIN WHY.
D) Find a general expression for the fugacity for this compound in the vapor/gas phase, as a function of temperature and pressure. For full credit, the equation should be an algebraic equation (not a differential equation) and should contain only P, T, f and constants.
E) Give your best estimates of the liquid phase fugacity and the vapor phase fugacity for this compound, each at T=550 K and P=20 bar.
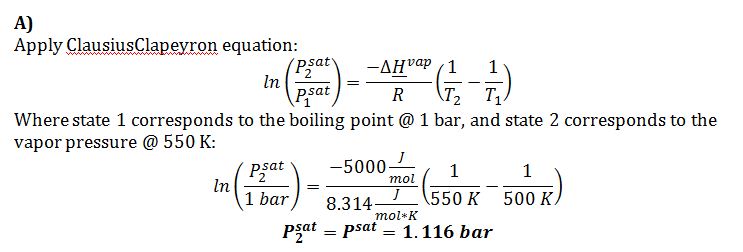
C)
The shortcut equation is only considered valid for calculations involving a reduced temperature within the range: 0.5
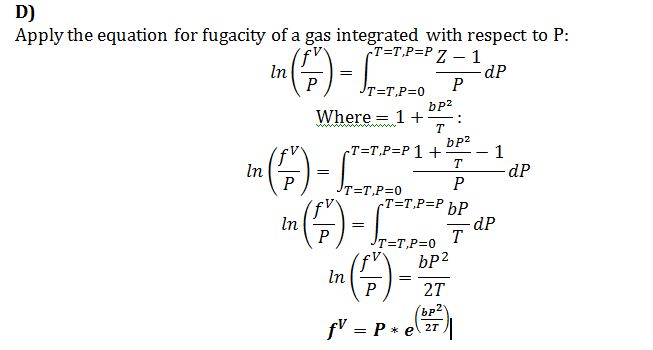
You might also like to view...
In the late 1970s and early 1980s, several companies specializing in _________________________ developed freestanding computer-drawing stations based on small independent computers called workstations.
Fill in the blank(s) with the appropriate word(s).
What is the best pH level to maintain in a water tower?
A) The pH of the water has very little effect on the operation of the cooling tower. B) Between 6.5 and 9 C) Lower than 6 D) Higher than 9
Udder evaluation has the highest weighting factor in determining dairy type.
Answer the following statement true (T) or false (F)
Samba is a utility that uses the ____ protocol, which is also used by Windows systems for sharing folders and printers.
A. RPC B. NFS C. IP D. SMB