If h = Planck's constant, the frequency of light emitted when an atom makes a transition is equal to
A. the energy lost by the electron divided by h.
B. the energy of revolution of the electron in its lowest energy orbit divided by h.
C. the energy gained by the electron divided by h.
D. the energy of revolution of the electron in its highest energy orbit divided by h.
Answer: A
You might also like to view...
The drawing below shows a sequence of Stern–Gerlach devices. By analogy to the cases discussed in the chapter, what do you think are the probabilities that an electron entering the last device will come out of that device’s plus and minus channels, respectively? 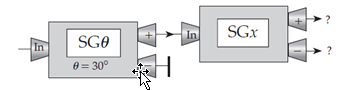
A. 1 and 0, respectively (AA if reversed, i.e., 0 and 1)
B. 0.933 and 0.067, respectively (BB if reversed)
C.  , respectively (CC if reversed)
, respectively (CC if reversed)
D. 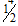 for both channels
for both channels
E. Some other probabilities (specify)
Which of the following will affect the vertical speed of a segment of rope that has a wave propagating along it?
1.The speed of a transverse wave along a rope. 2.The amplitude of a transverse wave along a rope. 3.The frequency of a transverse wave along a rope. 4.All of these.
In the H-R diagram, what are the two most important types of data plotted?
A) sizes and temperatures B) luminosities and masses C) absolute and apparent magnitudes D) apparent magnitudes and temperatures E) spectral classes and absolute magnitudes
Earth's magnetic field affects cosmic rays by
A) deflecting them. B) slowing their speeds. C) destroying them. D) absorbing them.