For the model you chose in part A, calculate the values for the adjustable parameters in the model (e.g., the value of A in the one-parameter Margules equation) at T=300 K.
This problem concerns mixtures of two liquids, W and Z. Some data for pure W and pure Z is given in the table below. No data on mixtures of these compounds is available, but based on their molecular structures, use of the ideal solution model is considered unrealistic. You are going to use a thermodynamic model to predict whether this pair of compounds will form stable liquid mixtures, or whether there will be a miscibility gap, at atmospheric pressure and various temperatures.

For the Scatchard-Hildebrand model:
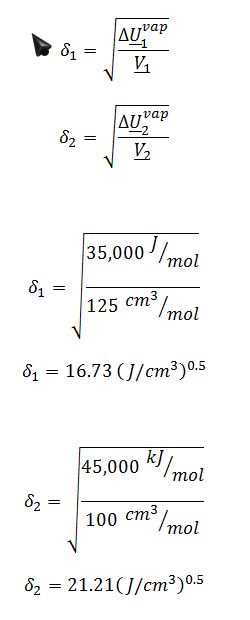
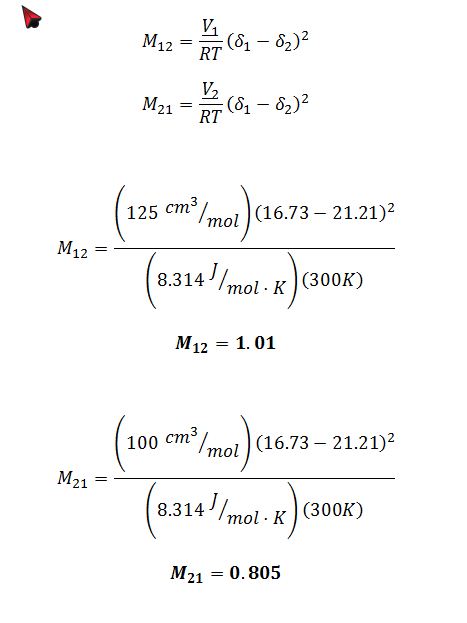
You might also like to view...
Describe what happens when excess air enters the furnace.
What will be an ideal response?
Using a textural triangle chart, it can be determined that a soil with 45% sand and 20% clay belongs to the ________ textural class
A) sandy clay B) sandy loam C) sandy clay loam D) loam E) loamy sand
Who is pivotal in preventing shop accidents by observing the rules of safety?
What will be an ideal response?
Technician A says many of the sensitive warning and informational sensors are placed at the front of the vehicle in the area of the grille and radiator support and on the rearview mirror. Technician B says some vehicles have an inertial shutoff switch that automatically turns the ignition off under certain operating conditions. Who is correct?
A. Technician A only B. Technician B only C. Both Technicians A and B D. Neither Technician A nor B