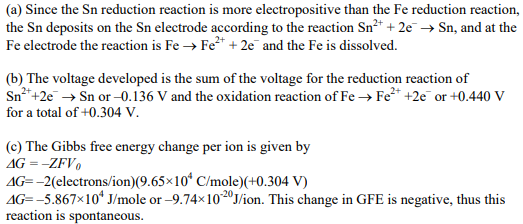
In an electrochemical cell, there is a tin electrode in a molar solution of Sn2+ ions and an iron electrode in a molar solution of Fe2+ ions.
(a) What reactions occur at the two electrodes?
(b) What voltage is developed in the cell? (c) What is the Gibbs free energy change per mole of ions involved in the reaction?
You might also like to view...
What do you notice about the relationship between the angular size of the galaxy and its redshift (how far the wavelength is shifted)?
A: The redshift tends to be larger if the angular size is smaller. B: The redshift tends to be smaller if the angular size is smaller. C: There seems to be no relationship between redshift and angular size.
A 0.40-kg mass, attached to the end of a 0.75-m string, is whirled around in a circular horizontal path. If the maximum tension that the string can withstand is 400 N, then what maximum speed can the mass have if the string is not to break?
a. 370 m/s c. 19 m/s b. 27 m/s d. 29 m/s
To observe a spectroscopic binary, we must be able to see both stars individually
Indicate whether the statement is true or false
Which of the following telescopes benefits most from adaptive optics?
A) the Keck I telescope on Mauna Kea B) the Hubble Space telescope C) the Arecibo radio telescope in Puerto Rico D) the Chandra X-ray Observatory