Which statement below is NOT an assumption made in the molecular model of an ideal gas?
A. The average separation between molecules is large compared with the dimensions of the molecules.
B. The molecules undergo inelastic collisions with one another.
C. The forces between molecules are short range.
D. The molecules obey Newton's laws of motion.
E. Any molecule can move in any direction with equal probability.
Answer: B
You might also like to view...
Elements in which the outer shell contains 8 electrons are known as the ______________
Fill in the blank(s) with correct word
A 40.0 liter gas tank is filled to the brim with gasoline when the temperature is 5.00 Gasoline has a coefficient of volume expansion of
Gasoline has a coefficient of volume expansion of 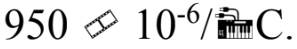 If the gas tank is moved into the sun of a hot summer day, the temperature of the gas tank is increased to 60.0
If the gas tank is moved into the sun of a hot summer day, the temperature of the gas tank is increased to 60.0  What is the volume of gasoline that overflows the tank (ignore the expansion of the gas tank)?
What is the volume of gasoline that overflows the tank (ignore the expansion of the gas tank)?
A. 3.95 liters B. 3.11 liters C. 2.67 liters D. 2.09 liters E. 1.55 liters
A thin copper rod 1.0 m long has a mass of 0.050 kg and is in a magnetic field of 0.10 T. What minimum current in the rod is needed in order for the magnetic force to balance the weight of the rod?
A) 9.8 A B) 2.5 A C) 1.2 A D) 4.9 A
Consider two identical and symmetrical wave pulses on a string. Suppose the first pulse reaches the fixed end of the string and is reflected back and then meets the second pulse. When the two pulses overlap exactly, the superposition principle predicts that the amplitude of the resultant pulses, at that moment, will be what factor times the amplitude of one of the original pulses?
a. 0 b. 1 c. 2 d. 4