Determine the standard Gibbs energy change of reaction at 325 K for the following gas-phase transformation:
P = 1 bar
The partial pressure at equilibrium due to component A is equal to 0.15 bar.
Relate equilibrium constant to partial pressures of each compound:
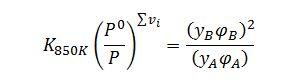
Assume the fugacity coefficients are both 1 (ideal gas condition).
By definition mole fraction is equal to partial pressure divided by total pressure.
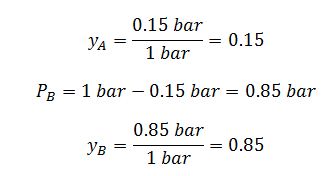
Since the reference pressure and total pressure are each equal to 1 bar, the equilibrium expressions simplifies to:
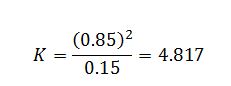
Evaluate equilibrium constant for the reaction:
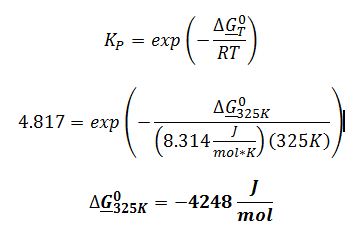
You might also like to view...
What can cause leakage and make the valve difficult to open or close?
A) Excessive force when opening or closing a valve B) Throttling a valve that is not designed for throttling C) Failure to clean valve stems D) Improper closing of a valve on a high-pressure line
Historically, farmers plowed their fields to prevent weeds. Today, most farmers use what technique instead of clear
plowing?
What will be an ideal response?The first step in the diagnostic procedure when attempting to solve an HVAC customer problem is _____
A) Verify customer concern B) Check for technical service bulletins C) Visual inspection D) Check for diagnostic trouble codes
Assuming that vs = 8cos(2t - 40°) V in the circuit shown in Fig. 11.38, find the average power delivered to each of the passive elements.
 FIGURE 1.png)