Explain the following statement: "In any natural process, some energy becomes unavailable to do useful work."
What will be an ideal response?
In any process, no energy is ever lost (it is always conserved). Rather, energy becomes less useful - it can do less useful work. As time goes on, energy is degraded, in a sense; it goes from more orderly forms (such as mechanical) eventually to the least orderly form, internal, or thermal energy. Entropy is a factor here because the amount of energy that becomes unavailable to do work is proportional to the change in entropy during any process.
You might also like to view...
Temperature variation of different parts of a person's body can be detected by analyzing the emission pattern of which type of electromagnetic radiation?
a. microwave c. x-rays b. infrared d. ultraviolet
A negatively charged rod is brought close to an uncharged electroscope. While the rod is close one's finger touches the far side of the metal ball on the electroscope. After the charged rod has been removed the finger is removed. The electroscope is
A. positively charged. B. uncharged. C. negatively charged.
Which of the following best explains what scientists think happened to outgassed water vapor on Venus?
A) Ultraviolet light split the water molecules, and the hydrogen then escaped to space. B) Water was removed from the atmosphere by chemical reactions with surface rock. C) It is frozen as water ice in craters near the poles. D) It turned into carbon dioxide by reacting with nitrogen in Venus's atmosphere.
The siphon shown is used to transfer liquid from a higher level to a lower level. If the fluid is drawn up and is continuous through the tube, determine the velocity of flow of gasoline if the vertical distance from the liquid surface to the outlet is 1.0 m
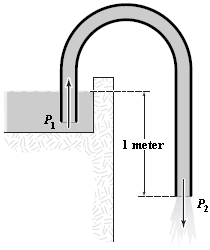
a.
1.1 m/s
b.
2.2 m/s
c.
4.4 m/s
d.
9.8 m/s
e.
6.5 m/s