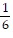Solve the problem.The pressure P, volume V, and temperature T of a gas are related by PV = n(RT + aP - bP/T). where a, b, n, and R are constants. For constant T, find dP/dV.
A. 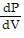 =
= 
B. 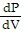 =
= 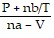
C. 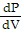 =
= 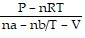
D.  =
= 
Answer: D
You might also like to view...
Answer the following statement(s) true (T) or false (F)
Many of the NSF-funded curriculum materials were designed to support teacher learning along with student learning because the curriculum developers recognized that implementing their written curricula was not trivial.
Answer the question.How can the graph of f(x) = -6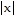 be obtained from the graph of
be obtained from the graph of 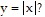
A. Stretch it vertically with factor -6 and reflect it across the y-axis. B. Stretch it vertically with factor -6 and reflect it across the x-axis. C. Stretch it vertically with factor 6 and reflect it across the y-axis. D. Stretch it vertically with factor 6 and reflect it across the x-axis.
Simplify.-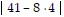 + 40 ÷ (6 - (-4)) - 32
+ 40 ÷ (6 - (-4)) - 32
A. 4 B. -86 C. -14 D. 31
Find the average rate of change for the function between the given values.f(x) = x3 + x2 - 8x - 7; from 0 to 2
A. -28
B. -2
C. 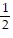
D. -