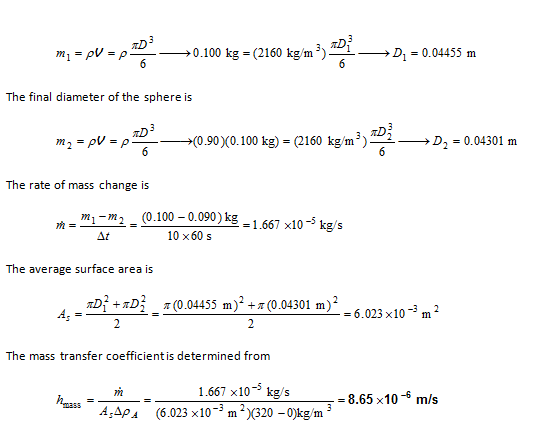
In an experiment, a sphere of crystalline sodium chloride (NaCl) was suspended in a stirred tank filled with water at 20°C. Its initial mass was 100 g. In 10 minutes, the mass of the sphere was found to have decreased by 10 percent. The density of NaCl is 2160 kg/m3. Its solubility in water at 20°C is 320 kg/m3. Use these results to obatin an average value for the mass transfer coefficient.
What will be an ideal response?
A sphere of crystalline sodium chloride (NaCl) was suspended in a stirred tank filled with water. The average mass transfer coefficient is to be determined.
Assumptions 1 The properties of NaCl are constant.
Properties The density of NaCl and its solubility in water at 20?C are given to be 2160 kg/m3 and 320 kg/m3, respectively.
Analysis The initial diameter of the sphere is
You might also like to view...
Which G-code program would be correct for a counter-clockwise arc with a starting point at (12.5,8.5), ending point at (19.5,8.5), and center at (16, 8.5):
A. G03 X16 Y8.5 I3.5 J0 B. G03 X19.5 Y8.5 I3.5 J0 C. G03 X16 Y8.5 I-3.5 J0 D. G02 X16 Y8.5 I3.5 J0
The data stream is a way of looking at the signals to and from the computer.
Answer the following statement true (T) or false (F)
A luminaire intended for installation in a pool or fountain structure's forming shell, where completely surrounded by water is referred to as ____.
A. a wet niche luminaire B. aquatic luminaire C. forming shell D. packaged source
A chiller typically cools ____________________ (what liquid?) for the purpose of air conditioning.
Fill in the blank(s) with the appropriate word(s).