The pump in the Rankine cycle raises the pressure of 12 kg/s of water from 20 kPa to 8 MPa. The boiler raises the temperature to 700°C, and the steam exits the turbine at a quality of 0.9, as sketched in the figure. Assume the pump and turbine are adiabatic.
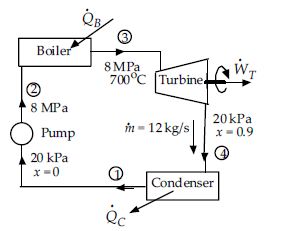
The heat input to the boiler is nearest:
A) 43.6 MJ/s
B) 39.2 MJ/s
C) 31.0 MJ/s
D) 24.8 MJ/s
A) 43.6 MJ/s
The rate of heat transfer demanded by the boiler is Q B = m ( h3 ? h2). This requires h2. The energy input to the water by the pump in a Rankine cycle is always very small compared to the energy requirements of the other components so it is always possible to neglect the pump energy in the cycle analysis and let h2 ? h1 = 251 kJ/kg (h f from Table C-2 at 0.02 MPa). The boiler heat requirement is then
Q B = m ( h3 ? h2 )
= 12 × ( 3882 ? 251) = 43 600 kJ/s or 43.6 MJ/s
You might also like to view...
What is CAM?
a. computer-automated manufacturing b. computer-aided machining c. computer-aided manufacturing d. computer-analyzing materials
Lizards are the smallest single group among the reptiles
a. True b. False Indicate whether the statement is true or false
List the primary reactor variables in effect on Rx-202.
What will be an ideal response?
What term describes the movement of electrons from one atom to another?
A) Energy transfer B) Electricity C) Chain reaction D) None of these