Solve the problem.In thermodynamics, the differential form of the internal energy of a system is dU = T dS - P dV, where U is the internal energy, T is the temperature, S is the entropy, P is the pressure, and V is the volume of the system. The First Law of Thermodynamics asserts that dU is an exact differential. Using this information, justify the thermodynamic relation 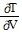 = -
= - 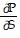 .
.
What will be an ideal response?
Answers will vary.
You might also like to view...
The function P, whose graph follows, gives the prime rate (the interest rate banks charge their best corporate customers) as a function of time for the first 32 wk during a year. Determine the values of t for which P is discontinuous.
?
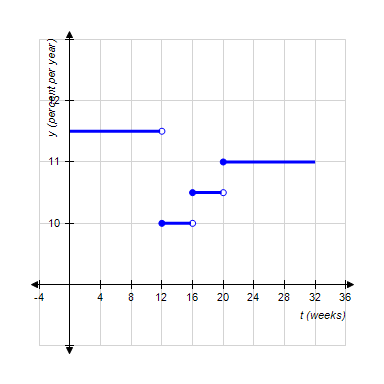
A. t = 11 B. t = 3, 2, 5 C. t = 8, 2, 5 D. t = 8, 16, 20
Determine the domain and range of the function.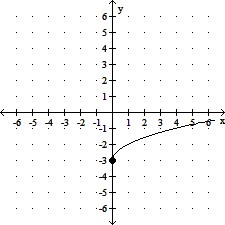
A. domain: [0, ?); range: [0, ?) B. domain: [0, ?); range: [-3, ?) C. domain: (-?, ?); range: [-3, ?) D. domain: [0, ?); range: (-?, ?)
State whether the function is a polynomial function or not. If it is, give its degree. If it is not, tell why not.f(x) = x3/2 - x6 + 7
A. No; x is raised to non-integer 3/2 power B. Yes; degree 6 C. Yes; degree 3 D. Yes; degree 3/2
Work the chain multiplication.(7)(6)(4)
A. 168 B. 764 C. 210 D. 17